The system, which mimics key features of gastrulating mouse embryos, reveals a mechanism that couples cell position and cell identity.
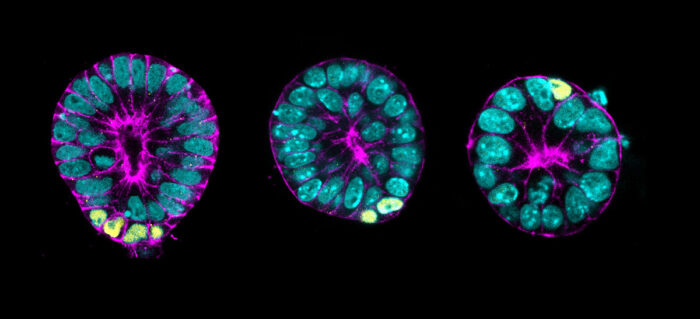
Marta Shahbazi’s group, in the LMB’s Cell Biology Division, has successfully established a three-dimensional embryonic stem cell culture system, which mimics key features of gastrulating mouse embryos. Using this, they have identified a mechanism which is crucial to ensure that the right cell types appear in the right place.
In early embryos, stem cells are pluripotent – meaning they have the ability to become any of the wide variety of cell types present in the final organism through differentiation. Thus, during development, embryonic cells need to decide their fate and identity and, crucially, make this decision at the correct time and place. This relies on a three-dimensional spatial awareness in order to sense the shape of the tissue they reside in and respond to the different cues from their surroundings. How exactly embryonic stem cells do this is not yet widely understood.
In part, this is because the developmental stage in which embryonic stem cells differentiate, known as gastrulation, is very challenging to study. In humans this occurs at 14 days post-fertilisation and in mice at 6.5 days. During gastrulation, embryonic stems cells change shape and move from their tissue of origin in a process called basal delamination. Analysing this at a molecular level is difficult as embryos are protected within the womb which makes them difficult to access and manipulate.
To overcome this, Marta’s group developed an in vitro 3D model consisting of self-renewing cells that recapitulates this developmental period. Nanami Sato and Viviane Rosa, postdocs in the group, conducted experiments using this system and demonstrated that cells undergo basal delamination in response to crowding, and this in turn triggers differentiation. Therefore, this mechanism ensures that all the cells that delaminate start to differentiate.
Specifically the group found that crowding triggers delamination of cells expressing high levels of the protein aPKC. Following this, these cells become more sensitive to Wnt signalling and increase expression of proteins such as the differentiation factor Brachyury, one of the key gastrulation players.
With assistance from the LMB’s transgenics facility, the accuracy of the in vitro stem cell model was validated using mouse embryos.
This research advances our understanding of embryonic development, and highlights the efficacy of using stem cell cultures to further investigate this process. Greater insight into embryonic development is integral to future research analysing the problems that can arise when conventional embryonic development is disrupted.
Beyond this, this research has further implications for furthering knowledge of basal delamination in other contexts. For example, in tumour development, metastasis whereby cancer cells spread to new parts of the body, can be initiated by basal delamination of cells. Some of the proteins examined within this study, such as the differentiation factor Brachyury, are also expressed in metastatic tumours. This raises the possibility that tumours reacquire embryonic pathways to increase aggressiveness.
This work was funded by UKRI MRC, UKRI EPSRC, EMBO, the European Research Council, the Wellcome Trust and the Japan Society for the Promotion of Science.
Further references
Basal delamination during mouse gastrulation primes pluripotent cells for differentiation. Sato, N., Rosa, VS., Makhlouf, A., Kretzmer, H., Sampath Kumar, A., Grosswendt, S., Mattei, A., Courbot, O., Wolf, S., Boulanger, J., Langevin, F., Wiacek, M., Karpinski, D., Elosegui-Artola, A., Meissner, A., Zernicka-Goetz, M., Shahbazi, MN. Developmental Cell
Marta’s group page