The human genome encodes thousands of proteins that are embedded in the membranes of all cells. These membrane proteins have numerous functions ranging from ion transport, to cell communication, to sensing odours, and others. In order to carry out these functions, they must be precisely oriented, folded, and assembled correctly. New research from Manu Hegde’s group in LMB’s Cell Biology Division has discovered a factor that is crucial for many membrane proteins to be oriented correctly when they are first made by the cell.
Nearly all membrane proteins are synthesised and assembled at the endoplasmic reticulum (ER), a labyrinthian membrane network inside eukaryotic cells. This is where all of the membrane-spanning segments of a protein are inserted and oriented correctly into the membrane so the protein can fold properly. While some proteins span the membrane only once, others are weaved in and out of the membrane numerous times. For example, G-protein coupled receptors (GPCRs), a large class of signaling molecules that control many aspects of human physiology, span the membrane seven times. How multi-spanning membrane proteins such as GPCRs are inserted correctly has not been clear.
Earlier genetic studies had found that disruption of a factor called the “ER membrane protein complex” (EMC) causes stress in the ER and altered levels of various multi-spanning membrane proteins. This raised the possibility that EMC might somehow participate in the production or fate of such proteins. To investigate this, Patrick Chitwood from Manu’s group isolated ER membranes from cells lacking EMC and tested whether they could support the correct insertion and assembly of a prototypical GPCR synthesised in a test tube. He found that around half as much GPCR was assembled properly with ER membranes lacking EMC than with ER membranes from normal cells.
When Patrick investigated where the process went awry, he found that the first membrane-spanning segment was inserted backwards in the absence of EMC. Several other GPCRs showed similar defects, albeit to different degrees, suggesting that EMC is generally needed to insert the first membrane-spanning segment of proteins whose N-terminus needs to face away from the cytosol. Membrane proteins whose first membrane-spanning segment needs to be in the opposite orientation don’t seem to need EMC for this step. When Patrick put purified EMC into synthetic membranes, the first membrane-spanning segment of a GPCR could be inserted correctly. Later steps require other factors such as the Sec translocation channel, but the details of how that works remain to be determined.
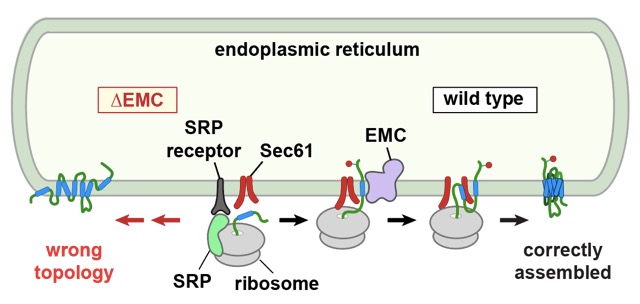
Earlier work from Manu’s lab had shown that EMC can also work as a stand-alone “insertase” for a class of simple single-spanning membrane proteins. The new work greatly expands this concept and suggests that EMC is a general-purpose membrane insertion factor that cooperates with previously established machinery to assemble complex multi-spanning membrane proteins. GPCRs, and membrane proteins in general, are of considerable medical importance, and understanding how cells make these proteins properly is an important goal of cell biology.
This work was funded by the MRC, the Cambridge Commonwealth, European and International Trust, and the Gates Foundation.
Further references:
Paper in Cell
Manu’s group page
Previous Insight on Research article: New machinery for membrane protein insertion