 The ubiquitin system is a complex system in all eukaryotic organisms involved in the regulation of most cellular processes. A huge variety of signals are assembled with ubiquitin molecules onto cellular proteins to mark them for a specific task. Important regulators of the ubiquitin system are deubiquitinating enzymes (DUBs), which remove, or cleave, ubiquitin chains in order to reverse these modifications. David Komander’s group in the LMB’s PNAC Division has a long-standing interest in the various types of ubiquitin signals. One such ubiquitin signal is a polyubiquitin chain linked via ubiquitin’s Lys11 residues. This chain type is important in the regulation of protein destruction, and in cell cycle processes. The only human DUB known to be specific for Lys11-linked ubiquitin chains is Cezanne. Using this example, they have for the first time followed a DUB throughout its catalytic life cycle. This has given unprecedented insight into the catalytic mechanism of a DUB and revealed a new mechanism of how ubiquitin chains can be cleaved. The new understanding of the catalytic process in deubiquitinases will become a key reference point for ubiquitin research.
The ubiquitin system is a complex system in all eukaryotic organisms involved in the regulation of most cellular processes. A huge variety of signals are assembled with ubiquitin molecules onto cellular proteins to mark them for a specific task. Important regulators of the ubiquitin system are deubiquitinating enzymes (DUBs), which remove, or cleave, ubiquitin chains in order to reverse these modifications. David Komander’s group in the LMB’s PNAC Division has a long-standing interest in the various types of ubiquitin signals. One such ubiquitin signal is a polyubiquitin chain linked via ubiquitin’s Lys11 residues. This chain type is important in the regulation of protein destruction, and in cell cycle processes. The only human DUB known to be specific for Lys11-linked ubiquitin chains is Cezanne. Using this example, they have for the first time followed a DUB throughout its catalytic life cycle. This has given unprecedented insight into the catalytic mechanism of a DUB and revealed a new mechanism of how ubiquitin chains can be cleaved. The new understanding of the catalytic process in deubiquitinases will become a key reference point for ubiquitin research.
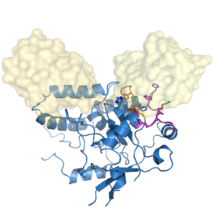
The research, initiated in 2010 and completed by Tycho Mevissen over the past three years in David’s group, determined the crystal structures of Cezanne at different steps during the catalysed reaction: of the enzyme alone, in complex with the substrate (Lys11-linked diubiquitin), and in complex with the product (monoubiquitin). To stabilise these complexes, they used chemically enhanced ubiquitin probes, which react with Cezanne to form a stable bond. They then performed different biochemical and biophysical experiments to probe the observed conformational changes and to characterise the reaction catalysed by Cezanne in unprecedented detail. Hydrogen-deuterium exchange mass spectrometry (HDX-MS) was used to show the intrinsic dynamics of the Cezanne protein, as well as to follow the impact of substrate and product complexes on these conformational dynamics. Collaborators at the Netherlands Cancer Institute and the High Energy Accelerator Research Organisation, Japan, provided important chemical tools and helped with synchrotron data collection, respectively. The research showed that in Cezanne large conformational changes that were entirely unexpected from previous studies were a key feature that determined Cezanne’s specificity.
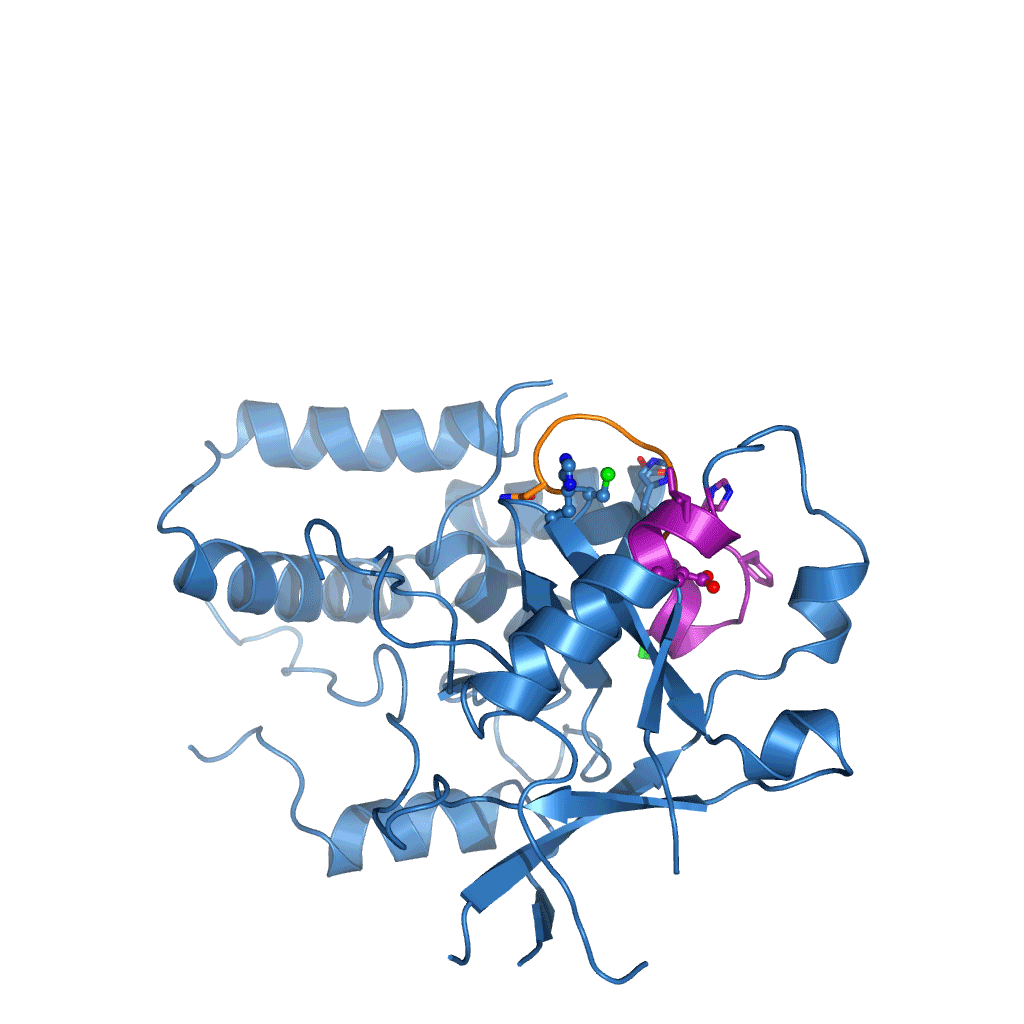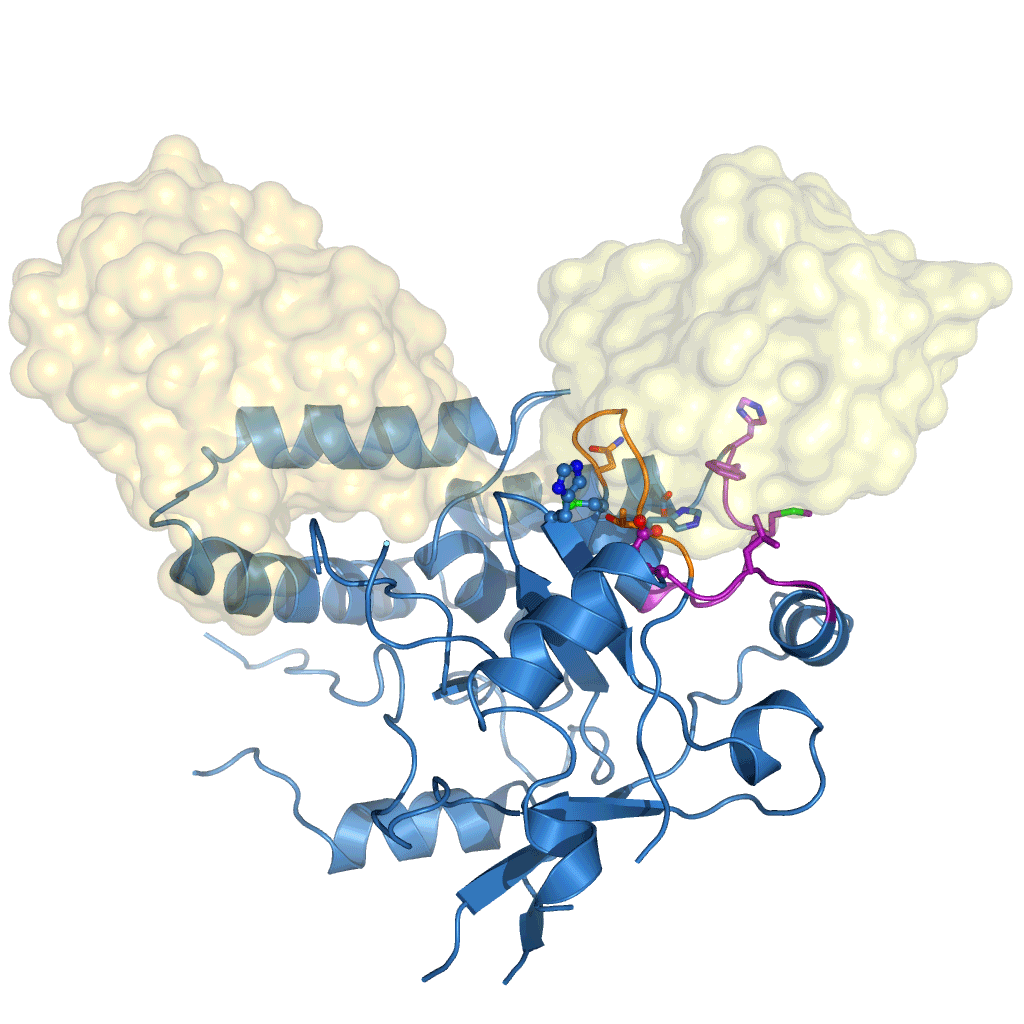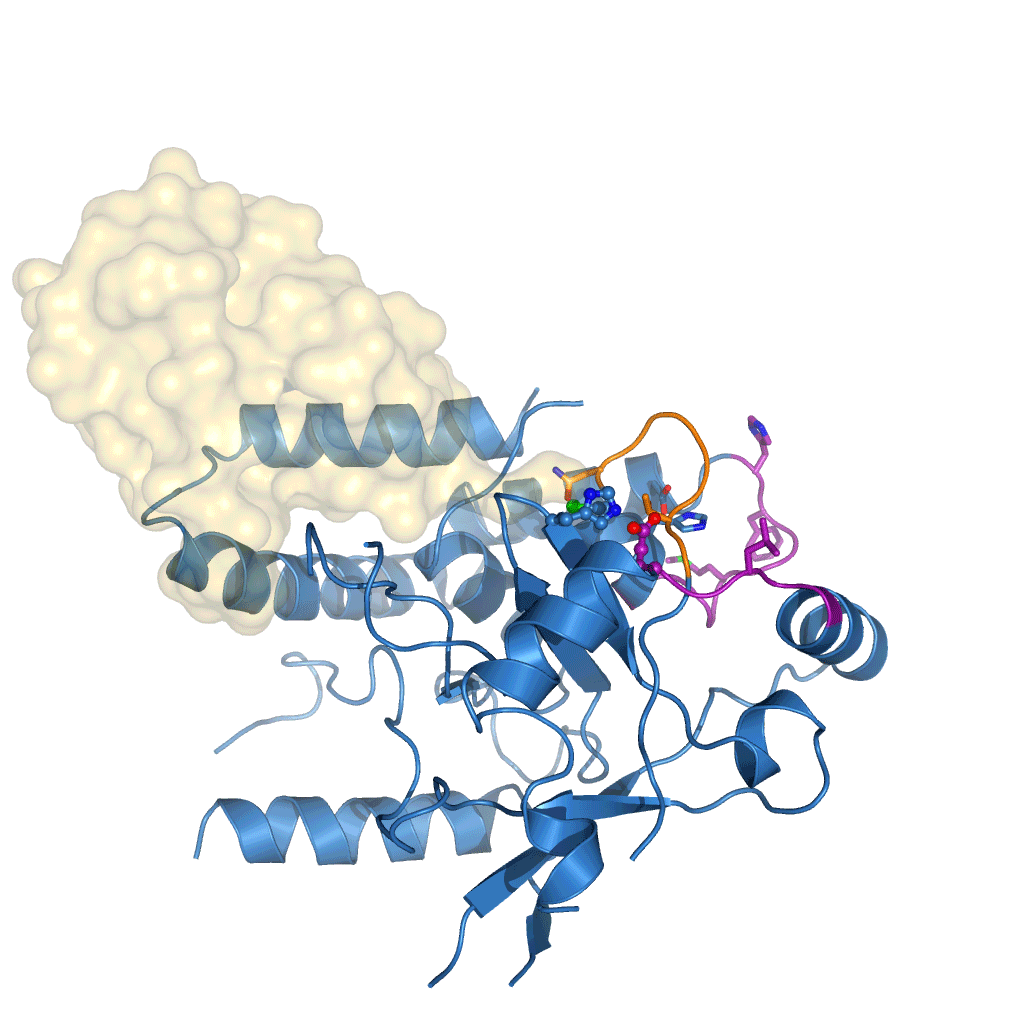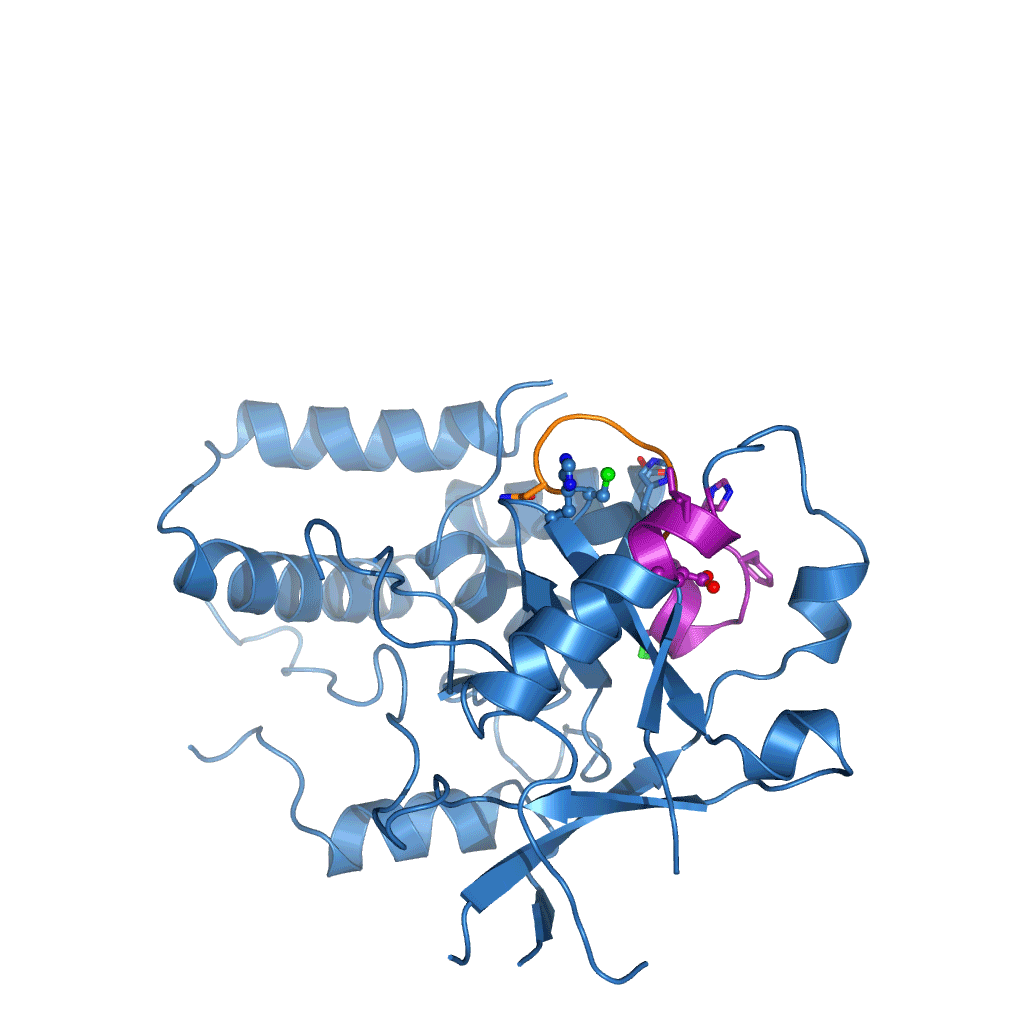 Cezanne is an important enzyme with roles in immune signalling, and it represents a potential drug target for the treatment of inflammation or cancer. The understanding of Cezanne’s conformational flexibility is essential for future endeavours to pharmacologically target Cezanne or related DUBs as these conformational changes need to be factored into the small molecule drug discovery process.
Cezanne is an important enzyme with roles in immune signalling, and it represents a potential drug target for the treatment of inflammation or cancer. The understanding of Cezanne’s conformational flexibility is essential for future endeavours to pharmacologically target Cezanne or related DUBs as these conformational changes need to be factored into the small molecule drug discovery process.
The work was funded by the MRC, the European Research Council, the Lister Institute for Preventive Medicine, the British Heart Foundation, a Netherlands Organisation for Scientific Research VICI grant, the Marie Curie ITN UPStream and Marie Curie and EMBO Long Term Fellowships.