The human genome encodes approximately 5000 membrane-embedded proteins that carry out many essential processes such as cell-to-cell communication, cell adhesion and intracellular trafficking. Almost all of these proteins are assembled at the endoplasmic reticulum (ER) by molecular machines that guide them into the membrane. Because these thousands of membrane proteins are highly diverse in size, shape and charge, different machines are needed for different types of membrane proteins. Alina Guna from Manu Hegde’s group in the LMB’s Cell Biology Division, in collaboration with Norbert Volkmar from John Christianson’s group at the University of Oxford, has discovered a new membrane insertion machine called the ER Membrane protein Complex (EMC).
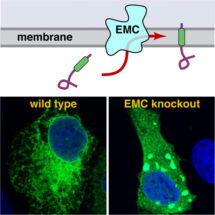
Manu’s group previously identified a mechanism by which a special type of membrane protein, called tail-anchored proteins, is inserted into the membrane. However, it was clear that some tail-anchored proteins did not use this pathway. In particular, Alina noticed that tail-anchored proteins whose membrane-embedded part of the protein is of medium to low hydrophobicity, did not use the known mechanisms to insert into the membrane. This suggested that some other previously unknown insertion machinery exists in the ER. A crucial clue to the identity of this alternative ‘insertase’ came from Norbert and John’s research. They had been studying EMC in cultured cells and noticed that when EMC was non-functional the cells could not properly make a particular tail-anchored protein called squalene synthase. This raised the possibility that EMC is somehow involved in inserting certain tail-anchored proteins into the membrane.
Alina monitored squalene synthase insertion into isolated ER membranes in a test tube and found that ER vesicles selectively lacking EMC could not insert squalene synthase properly. To determine whether EMC was responsible for directly carrying out insertion, Alina purified EMC, composed of ten different subunits, from mammalian cells and incorporated it into synthetic membrane vesicles. Squalene synthase could now insert into these EMC-only vesicles as efficiently as into natural ER membranes. This showed that EMC is both necessary and sufficient for the insertion of transmembrane domains.
Manu’s lab is now investigating how EMC performs its ‘insertase’ function, and whether this activity helps more complicated membrane proteins insert and assemble properly. Most drugs in clinical use target membrane proteins, highlighting their importance to human physiology. Methods to selectively modulate disease-released membrane proteins may benefit from an understanding of how they are correctly made and assembled inside cells.
This work was funded by the Medical Research Council, the Ludwig Institute for Cancer Research and Gates Cambridge.
Further references:
Paper in Science
Manu’s group page
John Christianson’s group page