Translating ribosomes display a beacon that is exploited for selective mRNA degradation
Cells tightly control the levels of ‘housekeeping’ proteins to maintain smooth operation of basic life processes. The most common way cells accomplish this task is feedback control of transcription to turn on or turn off genes in response to perceived need of their protein products. Another way is to degrade the messenger RNA (mRNA) intermediate between genes and proteins, so that less protein is made. Manu Hegde’s lab in the LMB’s Cell Biology Division has now discovered a protein used by cells to find the unneeded mRNA to trigger its destruction.
One group of proteins whose levels are controlled through mRNA degradation are tubulins. Almost 40 years ago, researchers noticed that cells seem to monitor how much free tubulin they have and adjust the levels of tubulin mRNAs in a process called autoregulation. Although it was understood that autoregulation occurred through control of mRNA degradation, no factors in this feedback process were ever discovered so it was unclear how it worked.
What are tubulins?
Tubulins are the proteins that join together, or polymerise, to form microtubules. Microtubules control key cellular events such as cell movement, cell shape, and cell division. The dynamics of microtubule polymerisation are highly dependent on the precise levels of tubulin, which probably explains why cells have evolved a mechanism to tightly control its mRNA levels.
Tubulins are critical in the structure and function of neurons and various neurodevelopmental diseases are caused by mutations in different tubulin genes. Tubulin is also the target of drugs used to treat gout and certain types of cancer. The discovery of a factor that regulates tubulin might in the future lead to new therapeutics for these diseases.
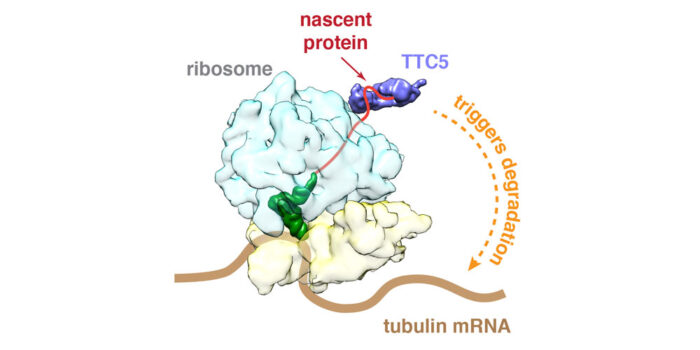
To investigate how cells regulate tubulin production, Zhewang Lin in Manu’s lab searched for factors that selectively engage ribosomes producing tubulin. He discovered a factor called TTC5 that binds only to ribosomes actively making tubulin. Zhewang then worked with Vish Chandrasekaran, in Venki Ramakrishnan’s group, in the Structural Studies Division, to determine the structure of TTC5 bound to a tubulin-producing ribosome. This showed that TTC5 has a groove within which the very beginning of tubulin binds as it emerges from the ribosome. Using this structure, Zhewang made individual mutations that prevent tubulin recognition and showed that cells containing such mutations in TTC5 completely lose their ability to regulate tubulin production rates in response to perceived excess free tubulin.
What happens when cells cannot fine-tune their tubulin content? To address this, Zhewang worked with Ivana Gasic in Tim Mitchison’s lab at Harvard. Ivana found that the precise alignment and segregation of chromosomes is more prone to errors in the absence of a functional TTC5. This is probably because these events during cell division depend critically on the precise orchestration of microtubule polymerization and depolymerization.
Additional experiments by Zhewang showed that TTC5 is not always available to engage tubulin-producing ribosomes. Instead, it is normally sequestered by a yet-unidentified inhibitor, which releases TTC5 only under conditions when cells detect too much free tubulin. The group is working to find this inhibitor and understand how it sequesters and releases TTC5 in a regulated manner.
The study illustrates how the nascent protein emerging from a ribosome is used as a homing beacon to broadcast where the encoding mRNAs are in the cell. In the case of tubulins, this beacon is exploited by TTC5 to find and selectively degrade tubulin mRNAs by recruiting mRNA degradation machinery that Manu’s lab is now working to find. The concept of using the nascent protein as a beacon to find the tubulin mRNA that encodes it represents an important mechanism of selective mRNA degradation. This mechanism may be used by cells to regulate the abundance of other key proteins that need to be maintained within a narrow range.
This work was funded by the MRC, the US National Institutes of Health, the Human Frontier Science Program, the Damon Runyon Cancer Research Foundation, Harvard Medical School, the Vallee Scholars Program, the Wellcome Trust, the Agouron Institute, and the Louis-Jeantet Foundation.
Further references
TTC5 mediates autoregulation of tubulin via mRNA degradation. Lin, Z., Gasic, I., Chandrasekaran, V., Peters, N., Shao, S., Mitchison, TJ., Hegde, RS. Science [Epub ahead of print]
Manu’s group page
Tim Mitchison’s group page
Sichen Shao’s group page