Discovery of a novel protein that binds and inactivates dynein motors for unimpeded delivery into cilia
The airways in our lungs are kept clear of mucus by the rhythmic beating motion of slender cellular extensions called cilia. This motion is known to be driven by dynein molecular motors, but it hasn’t been clear how the dyneins are delivered into the cilia after assembly in the cell cytoplasm. Using a unicellular ciliate model organism, Tetrahymena, Andrew Carter’s group, in the LMB’s Structural Studies Division, has discovered a novel protein, which they named Shulin, that packages these molecular motors for delivery.
Outer dynein arms (ODAs) are arranged along the cytoskeletal scaffold of cilia and pull on microtubules to make them slide and bend, thereby driving the cilia waving motion. Before they can perform this function, ODAs are first assembled in the cell cytoplasm and then must be delivered into the cilia. Genetic defects affecting ODA assembly or delivery can lead to a severe lung disease called Primary Ciliary Dyskinesia or to infertility, due to the role played by dyneins in the motion of sperm tails.
Understanding the molecular mechanisms involved in the delivery process is essential for the design of treatments for these conditions. Girish Mali, a postdoc in Andrew’s group, worked with the LMB’s Biological Mass Spectrometry and Proteomics and Light Microscopy facilities and collaborated with Juri Rappsilber’s group at Technische Universität Berlin to identify and characterise the regulatory factors which aid dynein delivery.
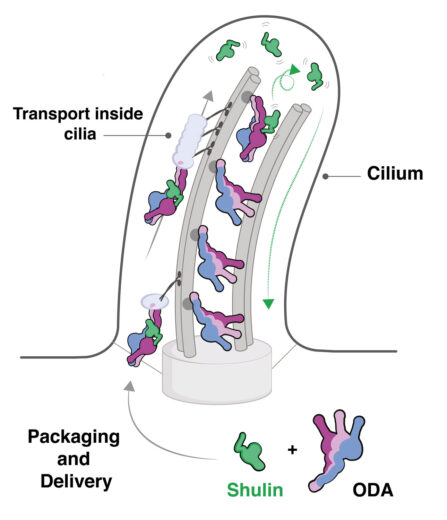
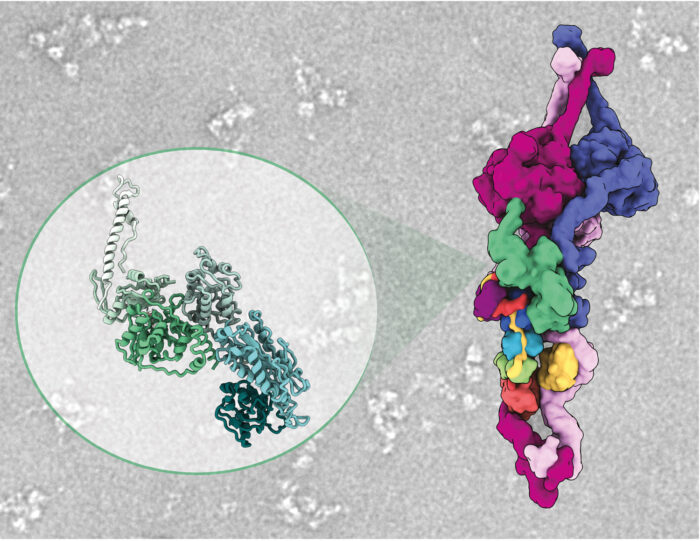
Purifying ODAs from the cytoplasm, Girish observed two additional factors bound to the ODAs. One of these factors was found to inactivate the ODA motor and lock its three arms together, so allowing unimpeded delivery of dyneins to the cilia. The team named this factor Shulin as it means “one that holds the trident” in Sanskrit.
Ferdos Abid Ali and Clinton Lau, also in Andrew’s group, helped determine a highly resolved cryo-EM structure of Shulin bound to ODAs to see how it held the arms together making this investigation span multiple scales of biology, from the level of single-celled ciliated organisms to the atomic scale.
Development of therapies for conditions caused by defects in ODA delivery or assembly will require a molecular understanding of the underlying causes. The identification of Shulin as a key protein that allows unimpeded delivery of dynein motors to the cilia is a step towards this. In addition, this study uncovers a novel inhibitory mechanism by which Shulin inactivates the dynein motor.
The work was funded by UKRI MRC, Wellcome Trust, and German Research Foundation.
Further references
Shulin packages axonemal outer dynein arms for ciliary targeting. Mali, GR., Abid Ali, F., Lau, CK., Begum, F., Boulanger, J., Howe, JD., Chen, ZA., Rappsilber, J., Skehel, M., Carter, AP. Science 371(6532): 910-916
Andrew’s group page
Juri Rappsilber’s group page