Discovery of a pathway responsible for recognising failures during assembly of multi-protein complexes in cells
Many proteins in our cells have to assemble together with other proteins to form large multi-protein complexes. These “molecular machines” (such as the spliceosome, ribosome, myosin, and proteasome) carry out some of the cell’s most important functions: splicing, translation, movement, and degradation. However, it is challenging for cells to make precisely the right number of individual proteins for a complete complex, so they often end up with “spare parts” or incomplete complexes that are not usable. Accumulation of unassembled or partially assembled proteins can be harmful and lead to disease, so cells have evolved methods to dispose of these intermediates. Now, Manu Hegde’s group, in the LMB’s Cell Biology Division, have identified a protein called HERC1 that performs this function. This study was in collaboration with Ana Narvaez when she was at AstraZeneca as part of the LMB’s and AstraZeneca’s joint Blue Sky venture to support pre-clinical research projects.
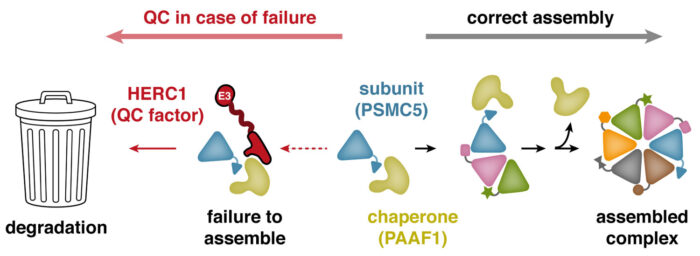
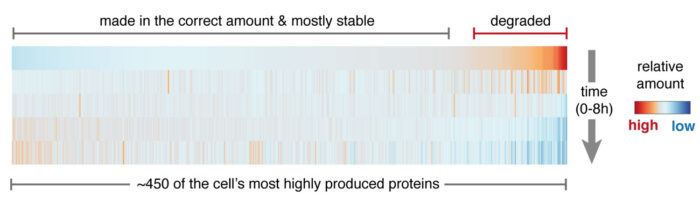
Manu’s group set out to find pathways that perform the quality control function of recognising and disposing of unassembled “orphan” proteins. Eszter Zavodszky, a senior scientist in the lab, worked with Sew-Yeu Peak-Chew from the mass spectrometry team to identify proteins in a breast cancer cell line that are rapidly degraded shortly after they are made. They reasoned that rapid degradation might be a sign that the protein was being produced in excess of what the cell needs, and could represent an orphan. Indeed, almost every one of the degraded proteins turned out to be a subunit of a larger complex. Eszter then examined some of these candidate orphan proteins further, eventually focusing on a protein called PSMC5, a component of a large structure called the proteasome. She discovered that unassembled PSMC5, but not the fully assembled proteasome, is recognised by a protein named HERC1. HERC1 is a ubiquitin ligase, a type of enzyme that tags proteins with ubiquitin molecules, which then serve as a flag to mark the protein for disposal.
After showing that efficient disposal of unassembled PSMC5 in cells depends on HERC1, Eszter investigated how HERC1 recognises PSMC5. To her surprise, she discovered that HERC1 does not recognise PSMC5 directly. Instead, HERC1 relies on PAAF1, a chaperone that helps PSMC5 during its assembly into the proteasome. Because PAAF1 detaches from PSMC5 once assembly is complete, it becomes clear why only unassembled PSMC5 is a target for HERC1. In this model, prolonged association of PAAF1 with PSMC5 indicates that assembly has failed, prompting HERC1 recognition and degradation of orphaned PSMC5. This new insight, that an assembly factor can also recruit degradation factors such as HERC1, might apply to many other protein complexes in the cell that rely on their own specialised chaperones for assembly.
Szymon Juszkiewicz, a postdoc in the Manu’s group, identified the specific region of HERC1 needed to recognise PSMC5, a challenging task given that HERC1 is almost 5000 amino acids long. This effort proved worthwhile because precisely that region is mutated in a mouse strain, found decades ago, that develops severe neurodegenerative disease. Eszter showed that this neurodegeneration-causing mutation is specifically impaired in recognising the PSMC5-PAAF1 complex. Thus, HERC1’s function in maintaining the correct balance of cellular proteins is especially important in non-dividing cells like neurons, which are particularly vulnerable to a build-up of unassembled or misfolded proteins.
Moreover, unassembled proteins are especially high in cancer cells because their mutated and rearranged genomes produce proteins in abnormal amounts. For this reason, cancer cells are particularly reliant on protein degradation, and drugs that block degradation are already used as anti-cancer treatments. By identifying the specific degradation pathways most relied upon by different types of cancers, it might be possible to devise more precise ways to prevent their ability to eliminate toxic unassembled proteins, leading to their demise. Therefore, identification of quality control factors such as HERC1 may provide new and more precise therapeutic targets.
This work was funded by UKRI MRC and supported through a research collaboration between AstraZeneca UK Limited and the Medical Research Council, reference BSF27.
Further references
Identification of a quality control factor that monitors failures during proteasome assembly. Zavodszky, E., Peak-Chew, S-Y., Juszkiewicz, S., Narvaez, A. J., Hegde, R. S. Science Vol. 373, Issue 6558.