Reverse electron transport causes overproduction of mitochondrial reactive oxygen species, a key contributor to the aggravation of tuberculosis pathogenesis
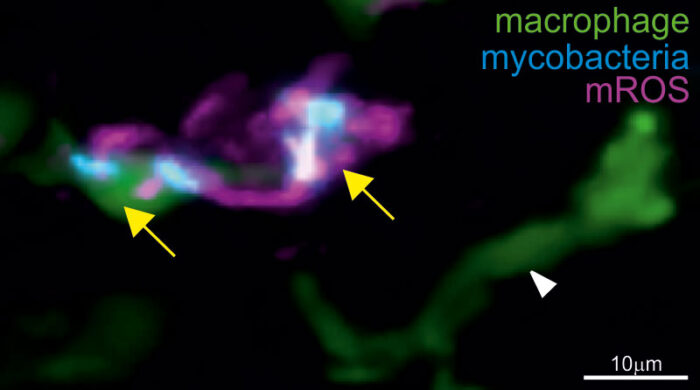
Tumour necrosis factor (TNF) is a key host protective factor against tuberculosis (TB). However, an excess of TNF is known to worsen TB pathogenesis, leading to more severe disease in TB patients. Lalita Ramakrishnan, Group Leader in the LMB’s Cell Biology Division and Head of the University of Cambridge’s Molecular Immunity Unit (housed at the LMB), has previously shown that excess TNF causes infected macrophages to die. This releases the infectious TB bacteria into the extracellular milieu thereby inciting further growth and disease progression. Her group has also shown that excess TNF caused TB-infected macrophages to overproduce mitochondrial reactive oxygen species (mROS), which causes them to die. Now, Lalita’s group has identified that TNF induces mROS, which drive pathogenesis and disease progression, through reverse electron transport (RET) in mitochondria during TB infection.
Normally, mROS are produced as electrons make their way down the electron transport chain along the cell’s inner mitochondrial membranes. Now, Lalita’s group have shown that pathological mROS in TB infected cells are not made through forward electron transport, but instead through RET, a pathway only recently recognised as pathologically important to conditions like heart attacks and strokes. This new study from Lalita’s group highlights the RET pathway’s role as a major pathogenic mechanism of TB too. They found that high levels of TNF caused levels of cellular succinate, a molecule involved in the Krebs cycle to increase, which, in turn, caused RET.
Francisco Roca, a former postdoc in Lalita’s group and now a Ramon y Cajal fellow at the University of Murcia, used zebrafish infected with Mycobacterium marinum, a close relative of human TB bacteria, to study TB. M. marinum is a natural pathogen of cold-blooded animals like zebrafish, causing TB-like disease. Zebrafish are transparent at early, embryonic stages, which allowed Francisco to visualise in detail the cellular and subcellular effects of infection and host genetic mutations on the progress of TB infection.
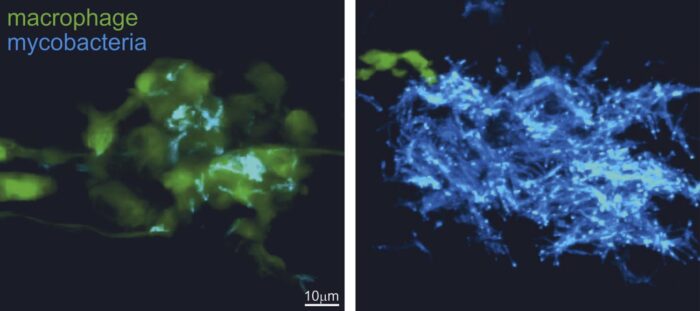
Zebrafish embryos were then treated through immersion in water containing pathway-inhibiting drugs. Francisco then used confocal microscopy to follow infection progression in real time. Laura Whitworth, another member of Lalita’s group, did further experiments on cultured human macrophages to confirm the utility of these disease-modifying agents, highlighting their potential to treat TB in humans. The study was supported by Hiran Prag, a postdoc in Mike Murphy’s group at the MRC Mitochondrial Biology Unit, who performed liquid chromatography-mass spectrometry quantification of succinate levels to demonstrate their increase when high levels of TNF were present.
This research highlights the importance of the RET-ROS pathway to the pathogenesis of TB. The group have already identified several existing drugs already in use in other disease contexts, which inhibit pathogenic macrophage death and thus could prove successful candidates for additional therapy to TB patients, including those with drug-sensitive or drug-resistant TB.
This work was funded by the Wellcome Trust, UKRI MRC, the National Institutes of Health (NIH), and the European Social Fund.
Further references
Tumor necrosis factor induces pathogenic mitochondrial ROS in tuberculosis through reverse electron transport. Roca, FJ., Whitworth, LJ., Prag, HA., Murphy, MP., Ramakrishnan, L. Science
Lalita’s LMB group page
Lalita’s University of Cambridge group page
Mike Murphy’s group page
A deeper understanding of how tuberculosis develops – Wellcome Trust video
MRC Mitochondrial Biology Unit news article
Previous Insight on Research articles
New cell death pathway in tuberculosis indicates potential use of commonly used drugs