New experimentally validated model reveals the key factors and steps used by cells to embed multipass proteins into their membranes
Around one-fourth of all human genes code for membrane-embedded proteins. Most of these proteins weave back and forth across the membrane multiple times, and are called multipass proteins. Some examples of the many multipass proteins include ion channels, signalling proteins, such as G-protein coupled receptors (GPCRs), and nutrient transporters. Despite their central importance for human physiology, it is unclear how they are correctly inserted into the membrane. New research from Manu Hegde’s group in the LMB’s Cell Biology Division, in collaboration with Robert Keenan’s lab at the University of Chicago, now sheds light on the fundamental problem of multipass membrane protein biogenesis.
For decades, the prevailing model has been that multipass proteins are weaved into the membrane iteratively, one transmembrane domain (TMD) at a time. In this model, each TMD successively passes through a lateral gate in the protein translocation channel called Sec61. Sec61 is well established as the channel for protein secretion, and experiments with single-TMD membrane proteins showed Sec61 can also work for their insertion. It has been assumed that multipass proteins also rely on Sec61’s lateral gate for insertion, but this was not tested experimentally. Manu’s group investigated this problem by studying the biogenesis of Rhodopsin, a multipass protein that detects light to enable vision. In earlier work, they discovered the chaperone, termed the PAT complex, that interacts with Rhodopsin as it is being inserted and folded. Although the PAT complex was shown to facilitate the biogenesis of Rhodopsin and several other multipass proteins, how it worked was not known.
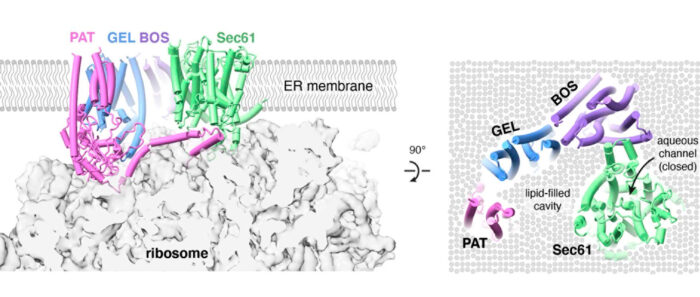
To address this, Manu’s group took a two-pronged strategy. Luka Smalinskaitė, a PhD student, used biochemical assays to investigate PAT complex function. In parallel, Min Kyung Kim, a postdoc, used electron cryo-microscopy (cryo-EM) to visualize the PAT complex during Rhodopsin insertion. Their combined efforts not only led to a better understanding of the PAT complex, but also suggested a completely new model for how multipass proteins are made. Luka discovered that the first TMD engages the PAT complex, which Min’s structure showed was positioned behind Sec61. Aaron Lewis, another PhD student, noticed that part of the PAT complex wedges between the ribosome and Sec61 and impedes Sec61’s lateral gate from opening. This observation was verified experimentally by Luka. The net effect of PAT complex engagement is to pull subsequent TMDs away from Sec61’s lateral gate, which is now impeded from opening. A parallel study led by the Keenan lab had found that additional factors, termed the GEL and BOS complexes, are recruited along with the PAT complex behind Sec61 to form the “multipass translocon”, which Min’s cryo-EM data supported.
Putting all of this together, the team proposed that the concave lipid-filled cavity region bordered by the PAT, GEL and BOS complexes could be the site where TMDs of a multipass protein insert and pack together into a correctly folded protein. This idea was supported by three findings. First, insertion of the second and third TMDs of Rhodopsin fails more frequently without the GEL complex, which is evolutionarily related to other TMD insertion factors studied previously by Manu’s group. Second, the third TMD of Rhodopsin was visualised by cryo-EM in this lipid-filled cavity immediately after its insertion. Third, inhibitors of the Sec61 lateral gate had no effect on biogenesis of several multipass proteins such as Rhodopsin. Yet, such inhibitors block translocation of long extracellular loops sometimes found in multipass proteins, indicating that the Sec61 channel is used to move long loops across the membrane.
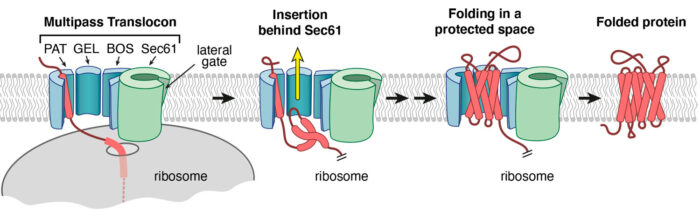
By defining the multipass translocon, and showing how it is assembled with roles for each of its parts, this research has revealed a new framework to explain how different segments of multipass proteins insert into the cell membrane. This occurs via different parts of a modular multipass translocon. One is an aqueous channel within Sec61 which is used to translocate long loops of membrane proteins (similar to how proteins are secreted). The second is a lipid-filled cavity formed by the other components of the complex which is used for insertion of TMDs two at a time. The PAT complex seems to control whether the translating protein is directed to the channel or the lipid-filled cavity.
Understanding how multipass proteins are properly made is vital because of their broad physiological relevance. Defects in the process can lead to various diseases such as retinitis pigmentosa, caused by incorrectly folded Rhodopsin, or cystic fibrosis, caused by an incorrectly folded ion transporter.
The work was funded by UKRI MRC.
Further references
Mechanism of an intramembrane chaperone for multipass membrane proteins. Smalinskaite, L., Kyung Kim, M., Lewis, A. J.O., Keenan, R.J., and Hegde, R. S. Nature. Oct 2022.
Substrate-driven assembly of a translocon for multipass membrane proteins. Sundaram, A., Yamsek, M., Zhong, F., Hooda, Y., Hegde, R.S., and Keenan, R.J. Nature. Oct 2022
Manu’s group page
Keenan Lab, University of Chicago
Nature News & Views – Assembly surprise for membrane proteins
Previous Insight on Research
Discovery of a chaperone for membrane proteins
New machinery for membrane protein insertion