Perforin-2 is found to be an effector of antigen escape in dendritic cells – a requirement for the activation of cytotoxic T cells
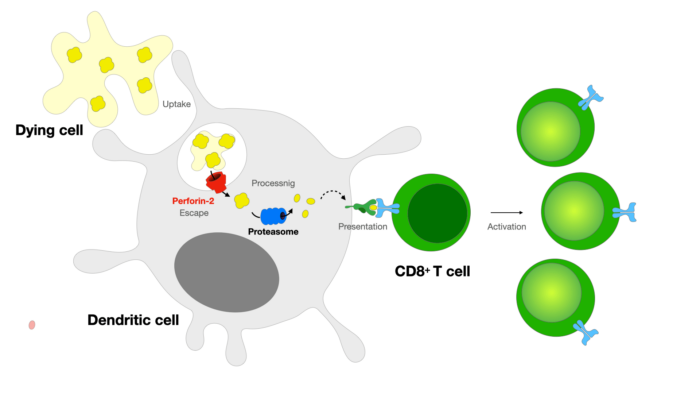
The immune system relies on cytotoxic T cells to efficiently kill infectious or cancerous cells. In order to prevent accidental killing of healthy cells, T cells circulate the body in a naïve state, where they do not express the molecules required for killing. T cell activation is carried out by dendritic cells, which can acquire antigens from infected and cancer cells and present them to naïve T cells. Dendritic cells transport the antigens into the cytosol, where they are processed to generate short peptides which are presented on MHC class I molecules to T cells. This process – known as cross-presentation – is critical for initiation of T cells to become cytotoxic in the response against tumours and certain viruses.
New research from Patrycja Kozik’s group in the LMB’s PNAC Division has discovered a new pathway of antigen transport from endosomes into the dendritic cytosol. Specifically, the group found that dendritic cells express a dedicated effector of escape – a pore-forming protein named perforin-2.
It had been previously noted that endocytic compartments in dendritic cells are unusually leaky and, as a result, a proportion of internalised proteins escape into the cytosol. This feeds exogenous proteins into the ubiquitin-proteasome degradation system and MHC class I pathway, resulting in antigen cross-presentation and priming specific T cells with antigens to target the present infected cells.
The identification of perforin-2, has provided a mechanistic explanation for the high efficiency of endocytic escape in dendritic cells, as the pores the protein creates deliver antigens into the cytosol.
To identify perforin-2, Pablo Rodríguez Silvestre, a former PhD student in Patrycja’s group, now a postdoc, developed a highly sensitive assay to monitor escape in individual cells. To do this, he used saporin – a toxin which is only active when in the cytosol. In a genetic screen, he looked for knockouts where saporin escape was no longer efficient and identified perforin-2 as the main hit.
Further experiments, with contributions from Marco Laub and Patrycja Krawczyk, PhD students in the group, found that when perforin-2 was genetically removed, cytosolic escape and antigen cross-presentation were impaired, leading to defective T cell responses.
The group’s study was supported by Georg Borner’s group at the Max Planck Institute of Biochemistry, who performed mass spectrometry experiments to help elucidate how perforin-2 cleavage is regulated by dendritic cells.
This research has helped further understanding of the mechanisms behind T cell mediated immune responses, which could present new avenues for future clinical research. Additionally, the work demonstrates that pore forming proteins can be used as transport channels without compromising the integrity of cellular membranes of viability of the cell altogether.
This work was funded by UKRI MRC, Boehringer Ingelheim Fonds, the Max Planck Society of the Advancement of Science, the University of Cambridge, Lister Institute, the Wellcome Trust, the Royal Society, and the UK Dementia Research Institute.
Further references
Perforin-2 is a pore-forming effector of endocytic escape in cross-presenting dendritic cells. Rodríguez-Silvestre, P., Laub, M., Krawczyk, PA., Davies, AK., Schessner, JP., Parveen, R., Tuck, BJ., McEwan, WA., Borner, GHH., Kozik, P. Science
Patrycja’s group page
Where’s Wally? How does the immune system identify cancer cells? – LMB Seminar for Non-Scientists by Patrycja Kozik
Georg Borner’s page