Disease-associated repetitive peptides physically block translocation of key motors along microtubule tracks within neurons, suggesting novel therapeutic strategies
Motor neurone disease (MND), also known as amyotrophic lateral sclerosis (ALS), is an adult-onset neurodegenerative disease with no known cure, symptomized by the decay of motor neurons in the spinal cord, brainstem and motor cortex. The most prevalent form of the disease is known to be caused by expansions of repeated DNA sequences in the gene C9orf72, although how these mutations lead to neuronal cell death is unclear. Simon Bullock’s group in the LMB’s Cell Biology Division has worked alongside scientists in several institutes including the Vlaams Instituut voor Biotechnologie (VIB), the University of Leuven (KU Leuven), the University of Edinburgh, and Stanford University to investigate how repetitive peptides produced from the mutated C9orf72 gene cause disease.
In many cells, simple diffusion can help distribute components around the cell. In contrast, the exceptionally long processes of neurons makes them reliant on active transport of organelles and macromolecules along microtubule tracks by molecular motors. Any breakdown in this cellular ‘railway system’ can therefore have damaging consequences on neuronal health. While studying neurons grown from MND patient stem cells, Laura Fumagalli, from Philip Van Damme’s group at VIB/KU Leuven, observed impaired axonal transport. Laura and Florence Young, a PhD student in the Bullock group, could induce the same effects in healthy human or fly neurons by exposing them to peptide repeats like those produced from the mutated C9orf72 gene.
Investigating the mechanism by which these peptide repeats impede transport, Steven Boeynaems (VIB/Stanford) and Laura found that they can associate with both the motors and the microtubule tracks. Florence then investigated whether these interactions were directly culpable for inhibiting transport. She used advanced microscopy to watch how purified motors move along microtubules bound to a glass surface when exposed to these peptides. She found that the peptides bind directly to microtubules through the unstructured ‘tail’ that extends from their outer surface. This, in turn, creates obstacles blocking the advancement of the key motors, dynein and kinesin.
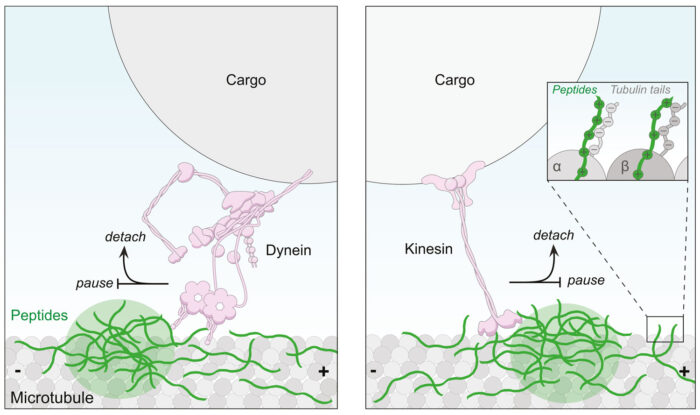
This study supports a direct inhibitory effect of repeating peptides produced from the C9orf72 gene on neuronal transport. In so doing, the work points to novel strategies for therapeutic intervention in C9orf72-associated diseases based on preventing peptides binding to microtubules or boosting the activity of the affected motors. However, because several mechanisms could combine to cause the disease, it may be necessary to apply these strategies together with those that target other cellular processes.
This work was funded by UKRI MRC, KU Leuven, Alzheimer Research Foundation, Flanders Impulse Program on Networks for Dementia Research (VIND), FWO Vlaanderen, the Agency for Innovation by Science and Technology, the European E-Rare-3 project INTEGRALS, ALS Liga België, the National Lottery of Belgium, EMBO, the Motor Neurone Disease Association, Alzheimer’s Research UK, and the Alzheimer’s Society.
Further references
C9orf72-derived arginine-containing dipeptide repeats associate with axonal transport machinery and impede microtubule-based motility. Fumagalli, L.*, Young, FL.*, Boeynaems, S.*, De Decker, M., Mehta, AR., Swijsen, A., Fazal, R., Guo, W., Moisse, M., Beckers, J., Dedeene, L., Selvaraj, BT., Vandoorne, T., Madan, V., van Blitterswijk, M., Raitcheva, D., McCampbell, A., Poesen, K., Gitler, AD., Koch, P., Vanden Berghe, P., Thal, DR., Verfaillie, C., Chandran, S., Van Den Bosch, L., Bullock, SL.**, Van Damme, P**. Science Advances *Joint first authors **Joint corresponding authors
Simon’s group page
Philip Van Damme’s page