Mechanistic explanation of how Fanconi anaemia factors prevent retrotransposon of LINE-1
All human proteins are encoded by just 2% of the human genome. When this was discovered upon the completion of the Human Genome Project, it was thought that the remaining 98% of human DNA was, in essence, ‘junk’. However, it is now understood that this DNA serves numerous functions crucial to life, but also can pose a threat. One such threat is retrotransposons – genetic elements likened to a ‘genetic parasite’ which can jump from one region of the genome to another, expanding over time to make up more and more of the genome. This has led to an arms race between the human host and the parasitic retrotransposon. Humans have evolved mechanisms to prevent retrotransposon expansion, while retrotransposons have developed strategies to evade these defences. New research from Nazareno Bona and Gerry Crossan, in the LMB’s PNAC Division, has identified one of these protections against retrotransposons.
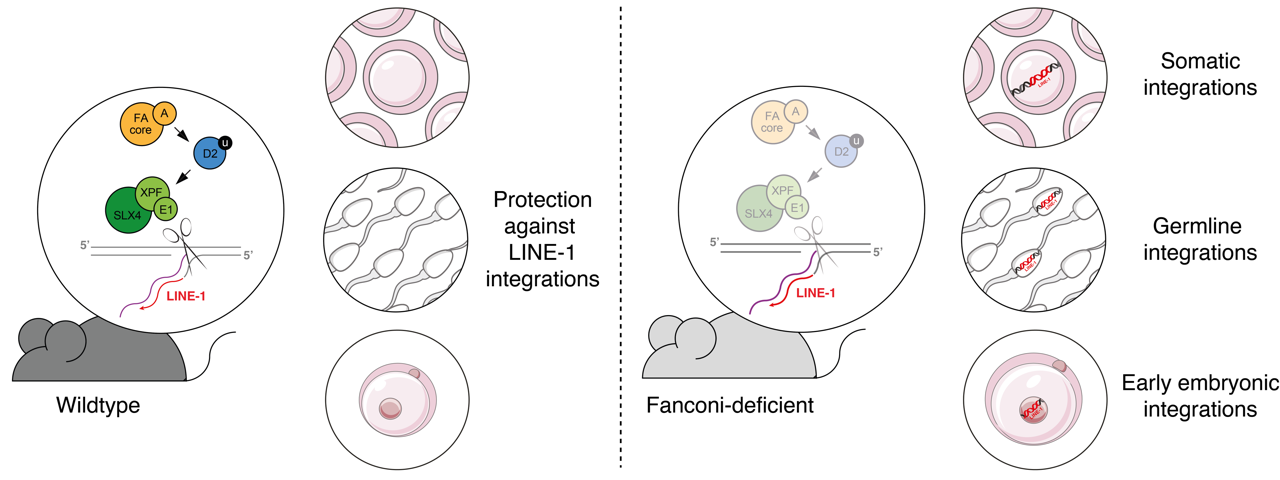
The pair studied long interspersed nuclear element 1 (LINE-1), and found that the host repurposes machinery usually used to repair damaged DNA to prevent the expansion of LINE-1 retrotransposons.
Specifically, they used genetically modified cells in tissue culture to determine that a family of proteins, defective in the rare human disease Fanconi anaemia, act together to prevent retrotransposition. After purifying host defence proteins, they found that the Fanconi anaemia factors were able to cut DNA at the site of a retrotransposon integration, and therefore prevent retrotransposons from jumping to a new site in the genome.
Further experimentation found that mice genetically modified to lack the Fanconi DNA repair pathway had extremely elevated rates of retrotransposon expansion. This also revealed that this mechanism of DNA repair is particularly critical to block retrotransposition at stages of development where other defences are inactive; notably in early embryonic development, and in sperm and eggs. This is the first demonstration that suppression of retrotransposition through DNA repair is physiologically relevant within living organisms.
These results hold clinical significance as LINE-1 retrotransposon expansion has been found in various human cancers. Notably, squamous cell carcinoma, a common form of skin cancer, has amongst the highest known numbers of retrotransposition events and is also very commonly found amongst patients with Fanconi anaemia. Uncontrolled retrotransposition may drive aspects of the Fanconi anaemia phenotype – which causes developmental defects, bone marrow failure, and a strong predisposition to cancers – and cancers in which DNA repair is inactive. Looking forward, it is possible that LINE-1 may be a significant therapeutic target.
This work was funded by UKRI MRC and the César Milstein Studentship from the Darwin Trust in Edinburgh.
Further references
Fanconi anemia DNA crosslink repair factors protect against LINE-1 retrotransposition during mouse development. Bona, N., Crossan, GP. Nature Structural & Molecular Biology
Gerry’s group page
Previous Insight on Research articles
How DNA damage induces appetite suppression and weight loss
Germ cells need DNA crosslink repair to develop normally