Cryo-EM analysis of the fungal GPCR Ste2 reveals a distinct activation method unique from other GPCR classes
G protein-coupled receptors (GPCRs) are membrane proteins which are essential for signal transduction and communicating with an organism’s surrounding environment. GPCRs are divided into six classes (A-F); Class D GPCRs are found exclusively in fungi and regulate fungal survival and reproduction. Previously, Chris Tate’s group in the LMB’s Structural Studies Division, determined the first ever structure of a fungal GPCR, using Ste2, a prototypical Class D1 GPCR which is essential for mating in the yeast Saccharomyces. cerevisiae. Ste2 was found to form a homodimer and couple to two G proteins simultaneously, which provided the first insights into how a transmembrane-mediated GPCR dimer could form and interact with G proteins. Now, new research from the group has shown that Ste2 has a novel method of activation which is unique from mammalian GPCRs.
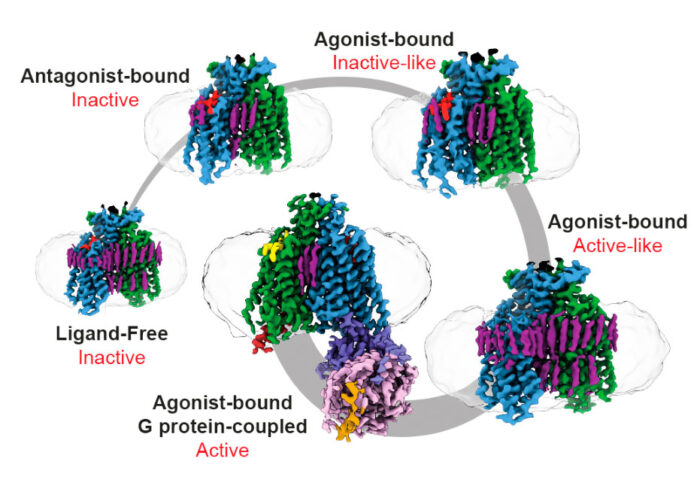
To capture Ste2 in its various conformations along the activation pathway, Vaithish Velazhahan, a PhD student in the Tate group, used different peptide ligands, purifying the GPCR separately under each condition. To purify ligand-free Ste2, Vaithish developed a method called pre-stabilisation of a GPCR by weak association (PSGWAY). This method involves adding a purified wild type heterotrimeric protein, Gpa1-Ste4-Ste18, to help stabilise ligand-free Ste2 before its purification. Then electron cryomicroscopy (cryo-EM) was used to collect multiple datasets of different samples, involving over 32,000 micrographs in total.
Computational processing of the datasets led to the determination of four new Ste2 structures; a ligand-free state, an antagonist-bound state, and two agonist-bound intermediate states. Their research highlights that Ste2 has a structural and activation mechanism distinct from all previously determined monomeric GPCRs. Using these structures, in collaboration with Nagarajan Vaidehi’s laboratory at the Beckman Research Institute, City of Hope, molecular dynamics simulations were performed which revealed allosteric communication pipelines that differed between the various states. The study provides intriguing possibilities for how allosteric communication within Ste2 affect their activation which is also relevant to understanding allosteric communication across other transmembrane-mediated GPCR dimers in humans.
This research into Class D GPCRs holds huge implications as fungal diseases, such as invasive candidiasis and pulmonary aspergillosis, presently a major disease burden worldwide, which also cause life-threatening conditions in humans. In addition, they can threaten global food supply through crop infection. An improved understanding of fungal GPCRs is vital to tackling these issues.
Furthermore, owing to their wide-ranging cellular functions, GPCRs are critical to drug development, with approximately one third of all FDA-approved drugs targeting them. Understanding the activation method of Ste2, as determined in this research and supported by the previous work on its structure, could encourage new possibilities for rational drug design. More specifically, from the findings of this work, it may be possible to target fungal GPCR signalling without also inadvertently targeting human GPCRs. This fungal-specific means of drug development, in theory, could help design medicines which avoid off-target effects and possible therapeutic side-effects – an important objective for successful drug development.
This work was funded by UKRI MRC, the Gates Cambridge Trust, and the National Institutes of Health.
Further references
Activation mechanism of the Class D fungal GPCR dimer Ste2. Velazhahan, V. Ma, N., Vaidehi, N., and Tate, CG. Nature.
Chris’ group page
Nagarajan Vaidehi’s group page
Previous Insight on Research
First structure of a fungal GPCR
High-resolution structure of a GPCR-arrestin complex