 Dyneins are a family of motor proteins that move along microtubules powered by chemical energy from ATP. Andrew Carter and his group in the LMB’s Structural Studies Division have solved the structure of a dynein protein bound to a chemical that mimics the shape of ATP, and have shown for the first time how the dynein can ‘walk’ along the microtubule.
Dyneins are a family of motor proteins that move along microtubules powered by chemical energy from ATP. Andrew Carter and his group in the LMB’s Structural Studies Division have solved the structure of a dynein protein bound to a chemical that mimics the shape of ATP, and have shown for the first time how the dynein can ‘walk’ along the microtubule.
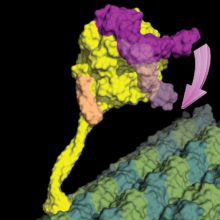
Dynein proteins carry various important cargos to different parts of the cell, and are crucial to correct cell function. When a bound molecule of ATP is split, it releases energy into the motor, causing it to change shape and to undergo a powerstroke, comparable to steam pushing a piston. Cycles of this drive the movement of dynein along microtubules in the cell, but exactly what happens during this process has been a long-standing question.
Helgo Schmidt and Ruta Zalyte, in Andrew’s group, have now provided the answer to this question. They used a small molecule to mimic ATP and trap the motor domain of human cytoplasmic dynein-2 at the point of its shape change and crystallised that complex to determine its 3D structure. The group developed a model for the mechanism of force production, and tested it by electron microscopy. They found that when the ATP is hydrolysed (split), a ring of ATPase domains in the protein closes. This closure pushes the linker domain into a state primed for force production, and also causes the dynein to release from the microtubule, so that it can move along it. Finding this mechanism is a major step forward in our understanding of how dynein works.
Defects in the dynein motor action are involved in a variety of diseases, for example viral infections, brain development defects and genetic disorders such as Jeune Syndrome, where mutations in the motor domain of human cytoplasmic dynein-2 cause defects in bone development. A better understanding of how dynein works might lead to new ideas for the treatment of these diseases. There may also be potential applications in the nanotechnology field for the development of artificial motors.
This work was funded by the MRC, the Wellcome trust and EMBO.