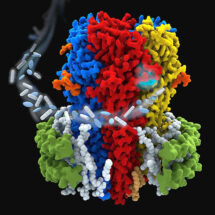
Practically all brain functions are controlled through a finely tuned balance of neuronal excitation and inhibition. The main inhibitory neurotransmitter in vertebrates is gamma-aminobutyric acid (GABA). GABA signals through two types of cell surface receptors: GABAA and GABAB, with GABAA receptors mediating millisecond-fast neurotransmission and GABAB receptors mediating slower signalling events. GABAA receptors were the last major class of human neurotransmitter receptors for which reliable structures were not available. Researchers in Radu Aricescu’s group in the LMB’s Neurobiology Division, with collaborators at Massachusetts General Hospital in Boston and VIB-VUB in Brussels, have used electron cryo-microscopy (cryo-EM) to determine the first structure of an intact GABAA receptor in a lipid bilayer.
GABAA receptors are molecularly diverse pentameric ion channels, that can be built from combinations of 19 different subunit types. This results in a vast number of different possible receptors with a range of functional properties. One of the most abundant forms found in vertebrate brains is the α1β3γ2 GABAA receptor which contains two α1, two β3, and one γ2 subunits. Radu’s group had previously solved a structure of a simpler, homo-pentameric, version in which all five subunits were β3, and more recently had reported a structure for a truncated heteromeric α1β3γ2 receptor, alongside similar reports from other groups. However, all these previous studies relied upon heavily engineered constructs and, in some cases, the experimental methods used to make the receptor suitable for analysis led to extensive damage to its structure.
In two back-to-back publications in Nature, Radu’s group has now overcome previous limitations to solve the first structure of an intact GABAA receptor, the human α1β3γ2. The receptor is visible in exquisite detail, in a lipid bilayer, and offers six different molecular snapshots. Each of these shows α1β3γ2 in complex with one of a group of small molecules that includes the benzodiazepines, diazepam (Valium) and alprazolam (Xanax). Working with protein purified from a cell line developed in Keith Miller’s lab at Massachusetts General Hospital, two researchers in Radu’s group, Simonas Masiulis and Duncan Laverty, used single-particle cryo-EM to solve the protein structures using “megabodies”, as developed by Jan Steyaert’s lab at VIB-VUB in Brussels, to stabilise the structures and allow high resolution models. These illustrate the binding modes for multiple clinical and research drugs targeting human GABAA receptors, and provide an unprecedented, in-depth understanding of how these receptors work.
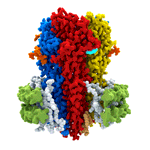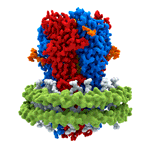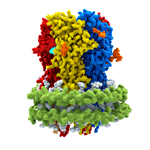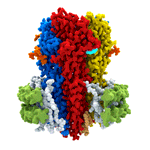
Dysfunction of GABAA receptors is associated with epilepsy, insomnia, depression, anxiety, and chronic pain, making them important drug targets. Indeed, a very large number of drugs in clinical use, including ones with anti-convulsant, anti-anxiety, analgesic, sedative, and anaesthetic properties, work via interaction with GABAA receptors. Some of these, such as benzodiazepines, entered clinical use decades before their target was known. However, as for most drugs, compounds acting on GABAA receptors can have unwanted side effects. With a greater understanding of the structure of drug targets, it might be possible to design better drugs. For example, in this work, the researchers found that Valium binds to two sites on GABAA receptors: at the major benzodiazepine site and also at a site that mediates the effect of general anaesthetics. This suggests the possibility to make specific changes to the structure of Valium that would alter its binding properties and could result in generation of a version that retains its calming and anti-anxiety properties, but without inducing sleepiness.
This work was funded by the MRC, BBSRC, Human Frontier Science Program, CRUK, Swiss National Science Foundation, National Institute for General Medical Sciences, the Department of Anaesthesia, Critical Care and Pain Medicine at Massachusetts General Hospital, INSTRUCT-ERIC, and the Research Foundation-Flanders.
Further references:
Cryo-EM structure of the human α1β3γ2 GABAA receptor in a lipid bilayer. Laverty, D., Desai, R., Uchański, T., Masiulis, S., Stec, WJ., Malinauskas, T., Zivanov, J., Pardon, E., Steyaert, J., Miller, KW. and Aricescu, AR. Nature
GABAA receptor signalling mechanisms revealed by structural pharmacology. Masiulis, S., Desai, R., Uchański, T., Serna Martin, I., Laverty, D., Karia, D., Malinauskas, T., Zivanov, J., Pardon, E., Kotecha, A., Steyaert, J., Miller, KW. and Aricescu, AR. Nature
Radu Aricescu’s group page
Keith Miller’s group page
Jan Steyaert’s group page
Nature News and Views: An in-depth structural view of a GABAA brain receptor