New screening system and Boolean logic gate circuits provide unprecedented insights into behaviour of type-2 innate lymphoid cells, a key contributor to allergic responses
Dysregulated behaviour by immune cells can cause allergic diseases, such as allergic asthma, hay fever and dust mite allergies. Andrew McKenzie’s group, in the LMB’s PNAC Division, previously identified that type-2 innate lymphoid cells (ILC2s) are one of these important cell types which drive allergy. However, details of how they are regulated have been challenging to elucidate owing to their rarity. Furthermore, using genetic methods to specifically target ILC2s has proved difficult.
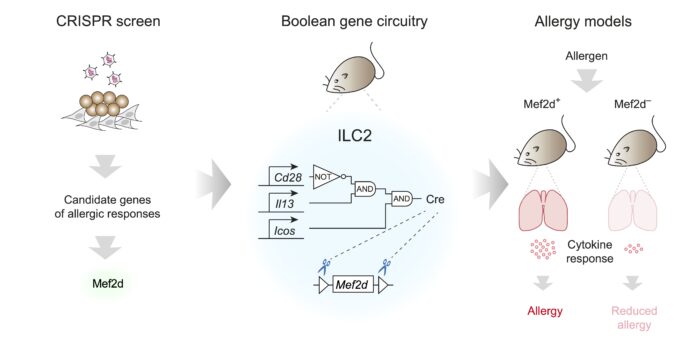
Spearheaded by Aydan Szeto and Paula Clark, and working with Patrycja Kozik (Group Leader in the LMB’s PNAC Division), the group used CRISPR to screen all transcription factors in the mouse genome for their function in ILC2s, amounting to over 1100 genes. This required developing a new cell culture system in which lymphoid progenitors from mice were expanded in vitro, before they were differentiated into ILC2s that mimic the process in vivo. This led to the discovery that the transcription factor Mef2d is a critical regulator of ILC2 function, including their expression of inflammatory cytokines which play key roles in asthma and allergies.
Next, to specifically study the importance of Mef2d in ILC2 function in vivo, the group took inspiration from Boolean logic gated circuits to construct similar gene circuitry in mice. Such circuits allow for the integration of different states using three main conditions (‘AND’, ‘OR’, and ‘NOT’) and output a binary value. The group identified three gene markers to discriminate ILC2s from similar cells. The final synthetic gene circuit was constructed using three pairs of molecular scissors (recombinases) and their unique cut-sites, introduced into the individual genes, so that their combined activity specifically defined and targeted ILC2s. Using this approach, the team, in collaboration with Padraic Fallon at Trinity College Dublin, Ireland, found that in multiple allergic models (e.g. to black mould), mice genetically deficient in Mef2d exhibited lower allergic responses.
Finally, in collaboration with Martin Knolle, a respiratory consultant at Cambridge University Hospital who recruited volunteers for the study, the group were able to demonstrate that ILC2s in humans also share similar gene pathways using Mef2d. This points to the future possibility of drug development to target Mef2d to combat allergic responses, an important goal given the high prevalence of allergic diseases.
This study represents the first time that a high-throughput genetic screening system has been used to study ILC2 function. The novel screening system identified a previously unappreciated role for Mef2d and could be used more widely to identify other new targets in allergic diseases. Furthermore, the Boolean logic circuitry in mice demonstrates how this approach could be applied to other fields including neuroscience and developmental biology.
This work was funded by UKRI MRC, the Wellcome Trust and the Croucher Foundation (via a Croucher Cambridge International Scholarship).
Further references
Mef2d potentiates type-2 immune responses and allergic lung inflammation. Szeto, A.C.H., Clark, P. A., Ferreira, A. C. F., Heycock, M., Griffiths, E. L., Jou, E., Mannion, J., Luan, S. L., Storrar, S., Knolle, M. D., Kozik, P., Jolin, H. E., Fallon, P. G., McKenzie, A. N. J. Science
Andrew’s group page
Previous Insight on Research articles
Neuroprotective protein ADNP is vital to immune reactions to allergens
Integrin-mediated T helper 2 cell clustering revealed as key step and potential therapeutic target in allergic immune response
Blocking action of intestinal immune cell enhances the immune response to colorectal cancer