A novel TRIM21-mediated mechanism of synergy between antibodies and T cells identifies viral nucleoproteins as important potential vaccine targets
Antibodies provide an important defence against viruses, often by binding to targets on the viral surface and stopping them infecting cells. Our immune system also generates a strong antibody response against internal virus proteins, including those that package the viral genome, known as nucleoproteins, but their role in clearing infection has not been understood. Leo James’ group, in the LMB’s PNAC Division, has revealed a new mechanism that explains the role of antibodies against nucleoproteins, which could have important implications for SARS-CoV-2 vaccination and treatment.
It has been known for many years that our immune system produces antibodies against viral nucleoproteins and measuring the presence of these antibodies has become a standard rapid diagnostic test to see if a patient has been infected by a certain virus, including for flu and SARS-CoV-2. However, the physiological role of these antibodies in immune defence has always been unclear.
Antibodies against nucleoprotein cannot directly stop viruses infecting cells in the way that those against the spike protein on the surface of SARS-CoV-2 can. For this reason, antibodies against nucleoprotein are known as non-neutralising antibodies. Interestingly, anti-nucleoprotein antibodies are often produced earlier during infection than those against surface proteins and non-neutralising antibodies play an important role in controlling infection by many enveloped viruses, including HIV and influenza. Leo’s group, along with their collaborators at ETH Zurich and the University of Oslo, wanted to investigate how these antibodies work and whether TRIM21, an antibody receptor discovered by Leo’s group to detect antibody-bound viruses inside our cells and drive their degradation, is involved.
To do this, Sarah Caddy and Marina Vaysburd from Leo’s group, together with Mark Wing in the LMB’s Biological Services Group, studied infection by an enveloped virus called LCMV (lymphocytic choriomeningitis virus) in mice. They found that anti-nucleoprotein antibodies helped the mice to recover and that TRIM21 and a type of T cell called cytotoxic or killer T cells were required for this recovery.
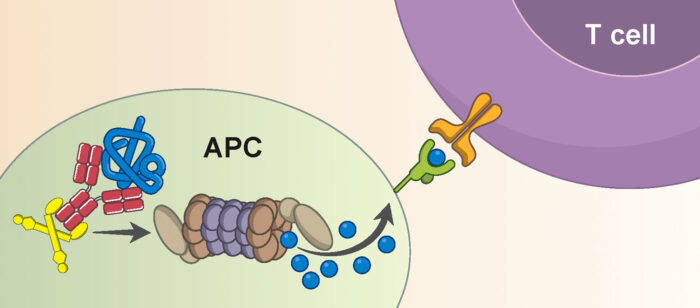
Cytotoxic T cells are important in viral defence as they kill any cell they recognise as being infected, diseased, or damaged. The novel mechanism that Sarah, Marina, and Mark identified involves TRIM21 recognising nucleoprotein via anti-nucleoprotein antibodies, leading to the viral protein being chopped up and displayed on the cell surface for detection by T cells. Activated cytotoxic T cells will then circulate the body killing infected cells, helping to clear the infection. This reveals a new mechanism of immune synergy between antibodies and T cells, which are often thought to work quite separately.
As this immune response involves T cells, one of the cell-types that mediate immunological memory, antibodies against nucleoprotein might stimulate long-term protection against future infection. Most work to develop COVID-19 vaccines has focused on the surface spike protein, but, as SARS-CoV-2 is an enveloped virus like LCMV investigated here, these findings suggest that vaccines could also be made using nucleoprotein, either alone or in combination with the spike protein.
However, this new mechanism could also be contributing to severe disease if patients fail to clear the virus but continue to mount an aggressive immune response. In this scenario, if most cells in the lungs are infected, then activation of killer T cells to attack these cells could leave insufficient healthy tissue for normal breathing. If this develops, blocking the activity of anti-nucleoprotein antibodies or TRIM21 function could help lessen the severity of disease. Therefore, this work provides multiple new concepts that could be exploited in the development of treatments for and vaccines against viral diseases like COVID-19.
The work was funded by UKRI MRC, Wellcome Trust, and Research Council of Norway.
Further references
Viral nucleoprotein antibodies activate TRIM21 and induce T cell immunity. Caddy, SL., Vaysburd, M., Papa, G., Wing, M., O’Connell, K., Stoycheva, D., Foss, S., Andersen, JT., Oxenius, A., James, LC. The EMBO Journal e106228
Leo’s group page
Annette Oxenius’ group at ETH Zurich
Jan Terje Andersen’s group at University of Oslo
Previous Insight on Research
TRIM21 senses infection and triggers immune response inside cells