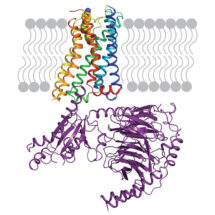
Scientists in Chris Tate’s group in the LMB’s Structural Studies Division have used electron cryo-microscopy to determine the structure of the serotonin receptor coupled to the heterotrimeric G protein Go, providing insights into how receptors bind specific G proteins.
Communication between cells throughout our bodies is vital for our health. Cells release signals, such as hormones and neurotransmitters, which must then be received and communicated to the inside of the recipient cell. In many cases this is achieved by G protein-coupled receptors (GPCRs) which are embedded in the cell’s outer membrane and bind the external incoming signal. Upon doing so, the portion of the GPCR inside the cell couples to and activates heterotrimeric G proteins which triggers intracellular signalling cascades, eventually resulting in cellular responses to the external stimulus.
The human genome encodes approximately 800 different GPCRs, collectively implicated in a wide range of physiological processes. However, there are only four families of G proteins (Gs, Gi/o, Gq, G12/13) to which the receptors couple, each one activating or inhibiting certain signalling pathways. With each human cell producing up to 120 different GPCRs and the limited diversity of heterotrimeric G proteins, there must be some particular way – a selectivity code – in which each receptor activates its specific G protein so that the appropriate cellular response to the external signal is achieved. Although there are atomic details of how the G proteins Gs couples to GPCRs, there was, until now, no such information on how receptors bind other types of G proteins – crucial for understanding the G protein-GPCR selectivity code.
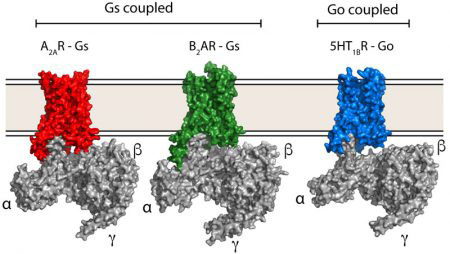
Scientists in Chris’ group purified the serotonin receptor (a GPCR) and combined it with the individual purified components of an engineered Go protein to recreate the signalling complex. They then added the small molecule donitripan, originally developed with the aim of treating migraines, which binds the serotonin receptor. This caused the GPCR to adopt its active state and bind Go. Pictures of the complex were then taken at very high magnification using electron cryo-microscopy which revealed the three-dimensional structure of the receptor-Go complex in molecular detail. This allowed the scientists to compare the structure of the Go-receptor interface to the previously elucidated Gs-receptor interface, providing insights into the G protein-GPCR selectivity code and how signalling specificity is achieved.
Serotonin is a chemical signal found in the central nervous system, the gastrointestinal tract and blood platelets, and contributes to the regulation of mood, appetite and sleep. Disturbances in serotonin signalling are implicated in certain psychiatric disorders and drugs targeting serotonin receptors, such as sumatriptan (Imigran ®), are used as anti-migraine agents. Understanding the activation mechanism of the serotonin receptor and the mode of binding of a failed anti-migraine drug, donitripan, will hopefully aid the development of new and improved drugs.
More broadly, GPCRs are the target of a third of small-molecule drugs treating a huge range of diseases, and it is crucial to understand in molecular detail how GPCR signalling works so that we can design better drugs. An essential component of this understanding is why any particular GPCR signals through a particular G protein pathway. Comparing the new Go-receptor structure to other GPCRs bound to different G proteins will build up a picture of why certain receptors preferentially bind only one G protein and why others bind multiple G proteins. The hope is that in the future it will be possible to design drugs that allow receptors to signal through only one pathway, which could make the drugs more potent and with reduced side effects.
This work was funded by the MRC, European Research Council and Heptares Therapeutics.