 During a viral infection, our immune system produces potent antiviral molecules which are hugely important for restoring us to health. However, if made at the wrong time these molecules can be damaging, leading to autoimmune diseases such as rheumatoid arthritis and multiple sclerosis. Our antiviral response must therefore be tightly controlled so that we are protected against infection but do not suffer from autoimmune disease.
During a viral infection, our immune system produces potent antiviral molecules which are hugely important for restoring us to health. However, if made at the wrong time these molecules can be damaging, leading to autoimmune diseases such as rheumatoid arthritis and multiple sclerosis. Our antiviral response must therefore be tightly controlled so that we are protected against infection but do not suffer from autoimmune disease.
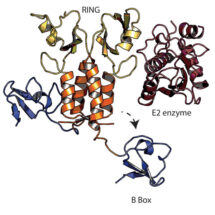
TRIM21, which is made in every cell in the body, is an important component of our body’s antiviral defence. When we are infected with a virus, TRIM21 recognises antibody molecules stuck to the virus, resulting in destruction of the virus and activation of immune responses. New work from Leo James’ group in the LMB’s PNAC Division has now revealed how TRIM21 is regulated so that it is only switched on at the right time.
Claire Dickson and Adam Fletcher from Leo’s group, along with Ji-Chun in David Neuhaus’ group in the LMB’s Structural Studies Division, used structural, biophysical and biochemical experiments to decipher how TRIM21 is regulated at the molecular level. TRIM21 contains a special element, a ‘B Box’, which is located in close proximity to TRIM21’s enzymatic domain and prevents it recruiting partner enzymes, thus keeping TRIM21 switched off. However, in the event of a viral infection the RING is phosphorylated causing TRIM21 to change in shape: the B Box moves away from the enzymatic domain, the partner enzyme can be recruited and thus TRIM21 is active. The researchers also showed that mutation of the specific part of the RING which is normally phosphorylated breaks regulation of TRIM21.
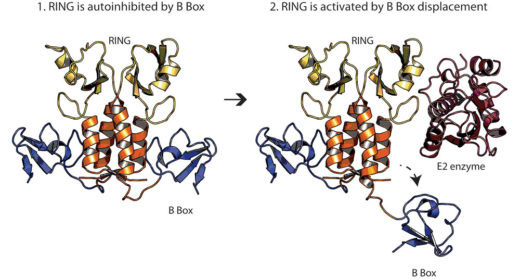
This work explains how TRIM21, part of the antiviral response, is regulated and may have implications for understanding disease and biotechnology. For example, the research suggests that dysregulation of TRIM21 could cause persistent immune signalling. Interestingly, TRIM21 is known to be involved in autoimmune diseases, such as lupus, therefore understanding more about TRIM21 is important for human health outside of viral infection. In addition, Leo’s lab has recently developed a new technique, ‘TrimAway’, which uses TRIM21 to selectively deplete proteins from the cell as an alternative to siRNA or CRISPR mutagenesis. Understanding how TRIM21 is regulated may be useful to improve the TrimAway method.
This work was funded by the MRC, the Wellcome Trust and a CJ Martin Fellowship.