Human eggs are frequently aneuploid, meaning they have the wrong number of chromosomes, and this is a major cause of pregnancy loss and Down syndrome. Aneuploidy in human eggs increases with advanced maternal age, which may explain why it is more difficult for women to get pregnant as they get older, and why miscarriages and Down syndrome are more likely in women of advanced age. However, the causes of this maternal age effect in humans have until recently been largely unclear. Work by Melina Schuh’s group in the LMB’s Cell Biology Division, in collaboration with Kay Elder and Martyn Blayney from the Bourn Hall Clinic, has shown that multiple changes in chromosome architecture could explain this effect.
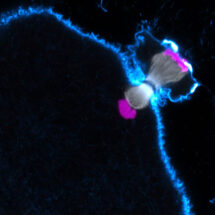
During the first meiotic division, eggs segregate entire chromosomes instead of sister chromatids as in mitosis. To this end, the two copies of each chromosome in the cell are joined with each other to form a single functional unit. The two microtubule attachment sites on each chromosome (kinetochores) that interact with the chromosome segregation machinery (spindle), become joined so that every unit of two chromosomes has two attachment sites only (and not four). Agata Zielinska, a PhD student in Melina’s group, used high resolution confocal microscopy of fluorescently labelled chromosomes, kinetochores and microtubules in live or fixed human oocytes (unfertilised eggs) to compare oocytes donated by young and older women. This revealed that the two supposedly fused kinetochores of each chromosome come apart in oocytes from older women, more than in young women, and interact independently with microtubules. The spindle then has problems in correctly handling the chromosomes, and chromosomes are often abnormally attached to the spindle. Unexpectedly, the chromosomes rotate on the spindle and orient as they normally would in mitosis, with each of their two attachment sites facing towards opposite spindle poles instead of facing towards the same spindle pole. Such rotated chromosomes were observed in around 40% of oocytes from women over 35. In addition, in oocytes from older women, the two copies of each chromosome frequently fell apart before anaphase (chromosome segregation) is meant to take place. This is problematic because the oocyte needs to eliminate half of the chromosomes before fertilisation, but cannot achieve this if the chromosomes are attached to the spindle in a rotated manner or have fallen apart prematurely. Oocytes seem to lack robust control mechanisms that can detect these abnormally attached chromosomes and delay anaphase onset until all chromosomes are correctly attached. This could contribute to the high rates of aneuploidy in human eggs.
This work reveals multiple changes in chromosome architecture in older eggs, which sheds light on why the quality of eggs deteriorates and could explain this maternal age effect. Indeed, some oocytes and their chromosomes are more than 40 years old at the time of fertilisation because oocytes in humans are present from birth onwards. Having identified these changes, we can now try to develop ways that allow us to prevent or correct for these age-related changes in chromosome architecture in order to decrease aneuploidy in eggs from older women and to increase their chances of having a family.
This work was funded by the MRC, an ERC starting grant and the Lister Institute.
Further references:
Paper in ELife
Melina Schuh’s group page
Bourn Hall Clinic, Cambridge