Trim-Away degrades proteins by directly ubiquitinating them, but this does not require lysine residues or free N-termini
Protein depletion is a powerful strategy to study protein function and treat disease. Unlike CRISPR and RNAi approaches that alter a cells genetic information or how it is translated, protein depletion methods act directly at the protein level. Trim-Away is a depletion technology developed by Dean Clift, Melina Schuh and Leo James at the LMB that allows widely-available, off-the-shelf antibodies to be used to degrade targets. Leo James’ group in the LMB’s PNAC Division has now identified surprising protein modifications that underly Trim-Away protein degradation technology, which may explain its broad applicability to deplete almost any cellular protein.
Trim-Away exploits the natural function of the intracellular antibody receptor TRIM21, which targets antibody-bound pathogens for degradation when they enter the cell. To deplete a protein using Trim-Away, researchers can simply choose an antibody that binds to their protein of interest and deliver this antibody to cells by giving them a mild electric shock. Once inside cells the antibody binds its protein target, forming a protein-antibody complex that is recognised by TRIM21 and destroyed within minutes. However, how TRIM21 triggers the degradation of an antibody-bound protein has not been shown until now.
What is ubiquitination?
Ubiquitin is a small protein that can be attached to other proteins through a process called ubiquitination, where it acts as a signal to recruit the cell’s protein degradation machinery. Ubiquitin is normally attached to protein lysine residues, but serine, threonine, cysteine and N-terminal ubiquitination can also occur. TRIM21 is a ubiquitin ligase enzyme, meaning that it can catalyse the attachment of ubiquitin molecules to proteins.
Previous work from Leo’s group showed that TRIM21 can ubiquitinate itself specifically at its N-terminus. This type of modification was thought to be important for TRIM21 function and also the function of other proteins within the TRIM protein family. To directly test if TRIM21 N-terminal ubiquitination is required for TRIM21 function, Leo Kiss in collaboration with Jonas Weidenhausen in Irmgard Sinning’s lab at the University of Heidelberg developed a method to modify the TRIM21 protein N-terminus with an acetyl group to prevent its self-ubiquitination. Indeed, Leo found that this N-terminally acetylated TRIM21 was no longer degraded in cells because it could not ubiquitinate itself. Surprisingly, however, in infection and Trim-Away experiments carried out together with Tyler Rhinesmith, he found that the N-terminally acetylated TRIM21 was still able to efficiently degrade antibody-bound cellular proteins and invading pathogens.
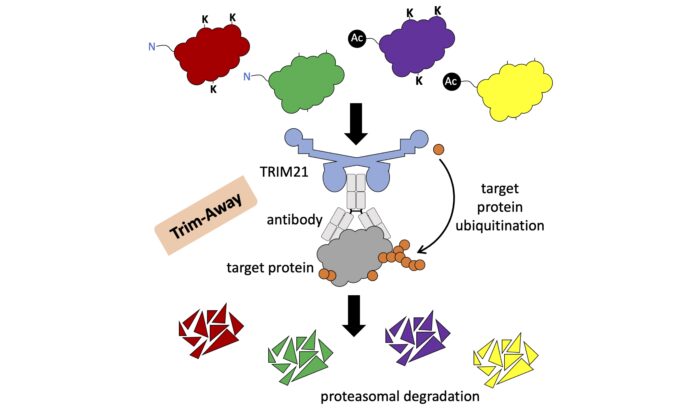
Reconstituting Trim-Away in vitro revealed that, in addition to ubiquitinating itself, TRIM21 can also directly ubiquitinate antibody-bound proteins. Furthermore, proteins targeted by Trim-Away inside living cells were found to be ubiquitinated prior to their degradation. The finding that Trim-Away degrades proteins by directly ubiquitinating them led the researchers to ask which residues ubiquitin is attached to. Surprisingly, they found that if they removed all lysine residues from a protein and blocked its N-terminus by acetylation the protein was still efficiently degraded by Trim-Away.
The authors speculate that TRIM21 may have evolved a promiscuous ubiquitination mechanism to remain one step ahead of rapidly-evolving pathogens that can easily mutate residues normally prone to ubiquitination. This mode of action may also explain why Trim-Away technology can be used to degrade a wide-range of cellular proteins.
This work was funded by UKRI MRC, Boehringer Ingelheim Fonds and Wellcome Trust.
Further references
Trim-Away ubiquitinates and degrades lysine-less and N-terminally acetylated substrates. Kiss, L., Rhinesmith, T., Luptak, J., Dickson, CF., Weidenhausen, J., Smyly, S., Yang, JC., Maslen, SL., Sinning, I., Neuhaus, D., Clift, D., James, LC. Nature Communications
Leo’s group page
Previous Insights on Research
Understanding TRIM21 activation allows Trim-Away toolbox expansion
TRIM21 is both enzyme and substrate when creating a signal to degrade bound viruses and proteins
TRIM21 links antibody and T cell immunity to combat viral infection