The first descriptions of HIV-1 matrix protein structures from within both immature and mature authentic virus particles show unexpected rearrangement on maturation
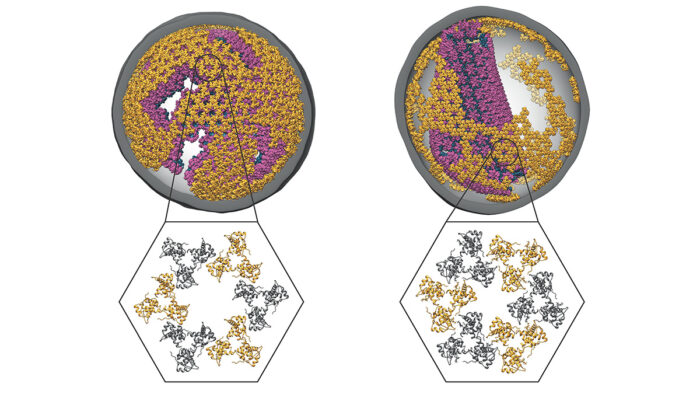
Human immunodeficiency virus type 1 (HIV-1) can cause persistent infections and result in acquired immunodeficiency syndrome (AIDS). A better understanding of the mechanisms of fundamental viral functions might be the key to finding new and more effective treatments for this disease. John Briggs’ group, in the LMB’s Structural Studies Division, has now provided the first description of HIV-1 matrix protein structures determined within both immature and mature authentic virus particles, showing how they rearrange on maturation of the virus ahead of infection of another cell.
HIV-1 virus particles have at their centre their RNA genome, which is tightly bound to nucleocapsid proteins and surrounded by a conical capsid composed of a large number of copies of the capsid (CA) protein. Around the capsid is the matrix, made of matrix (MA) proteins, and the viral envelope, a lipid bilayer membrane. Assembly of the virus particle involves a maturation step that prepares the virion for entry into another cell. Although much is known about this process and the structure of HIV-1 MA was first determined in 1994, the organisation of MA in intact viruses, and any rearrangements with maturation, had not yet been seen.
To study MA in intact HIV-1 virus particles, John’s group collaborated with Hans-Georg Kraüsslich’s and Barbara Müller’s groups at Heidelberg University Hospital and Carsten Schultz’s group at EMBL Heidelberg and Oregon Health & Science University. Using electron cryo-tomography (cryo-ET), the team could look at the proteins inside HIV-1 particles purified by members of Hans-Georg’s and Barbara’s labs.
Solving the structure of MA was made difficult by its small size, irregular packing, and proximity to the viral membrane. Kun Qu, a member of John’s group, overcame these challenges and found that MA rearranges between two different lattice organisations upon maturation. In the mature form, a lipid extends from the viral membrane into a pocket in MA. This suggests that HIV-1 maturation not only assembles the viral capsid, but also prepares the membrane-bound matrix for some role in cell entry or after infecting another cell, and it alters the viral lipid bilayer. This unexpected rearrangement could be responsible for clustering or activation of proteins on the viral surface or might in some way affect the virus particle’s ability to fuse with the membrane of a cell during infection.
Despite many years of intensive study, there is currently no vaccine against HIV-1 and it continues to be a major global health burden. These MA structures provide a basis for interpreting and designing further investigations into virus assembly and maturation in the ongoing search for novel antiviral strategies.
The work was funded by UKRI MRC, ERC under the European Union’s Horizon 2020 research and innovation program, EMBL, Deutsche Forschungsgemeinschaft, and NIH.
Further references
Maturation of the matrix and viral membrane of HIV-1. Qu, K., Ke, Z., Zila, V., Anders-Össwein, M., Glass, B., Mücksch, F., Müller, R., Schultz, C., Müller, B., Kraüsslich, H-G., Briggs, JAG. Science 373(6555): 700-704
John’s group page
Hans-Georg Kraüsslich’s group page
Barbara Müller’s group page
Carsten Schultz’s page
Science Perspective: Maturation of HIV-1