Parkinson’s disease (PD) is a neurodegenerative condition caused by the loss of dopaminergic neurons in the midbrain, which manifests clinically in the form of characteristic motor defects. Most PD cases are sporadic and found in people above the age of 60. However, roughly 10% of PD cases are autosomal recessive juvenile forms (AR-JP), causing early-onset PD. It is known that mutations in PARK genes are responsible for this, but often a molecular explanation is lacking. Research in David Komander’s group, in the LMB’s PNAC Division, with collaborators at the VIB-VUB Centre for Structural Biology in Brussels, Belgium, has now provided new insights, revealing how mutations in PINK1, one of the first PARK genes to be identified, leads to early onset PD.
Despite its importance, a structure of PINK1 had not been determined. The protein is a highly divergent protein kinase, which prevented modelling based on known kinase structures. PINK1 is a key regulator of the turnover of damaged mitochondria, and research over the last 4 years in David’s and other labs has shown that PINK1 attaches phosphate groups (‘phosphorylates’) to two substrates, namely ubiquitin as well as the ubiquitin-like domain of Parkin, another PARK gene. Curiously, the phosphorylated sites in these substrates appeared to be inaccessible to phosphorylation.
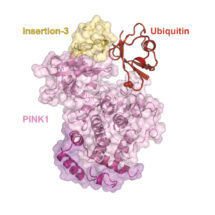
In order to provide a detailed understanding into PINK1-caused early-onset PD, Alexander Schubert in David’s group reasoned that he would need to generate a structure of PINK1 bound to ubiquitin. Such kinase structures with substrates bound are exceedingly rare.
Alex succeeded, and has now revealed what PINK1 looks like, how it is regulated, and how it accesses the inaccessible phosphorylation site in ubiquitin. Using NMR studies, with the help of the LMB’s NMR facility, the team first discovered a new conformation of ubiquitin that could be stabilised in a form ready for phosphorylation. To further trap the kinase in the transient ubiquitin phosphorylation event, they enlisted the help of the European research initiative INSTRUCT, who raised a nanobody against the complex. The llama nanobody stabilised the complex and allowed Alex to determine a crystal structure of PINK1 bound to its substrate ubiquitin.
The complex structure illuminates the molecular basis for more than 40 PD-linked mutations in PINK1 and explains more globally how kinases can access poorly accessible phosphorylation sites in substrates. This work offers new concepts for protein kinase research, as it also shows new ways for how these enzymes, major drug targets in their own right, bind to their substrates. This first active PINK1 structure may enable the rational design of small molecule activators that have been proposed to help patients with PD.
This work was funded by the MRC, the European Research Council, the Michael J. Fox Foundation and the Lister Institute for Preventive Medicine.
Further references:
Paper in Nature
Previous Insight on Research article: How phosphorylated ubiquitin activates Parkin
Previous Insight on Research article: Work on ubiquitination reveals insights into disease