Link found between tumour-related expression of the IL25 gene, innate lymphoid cells and reduced survival amongst colorectal cancer patients
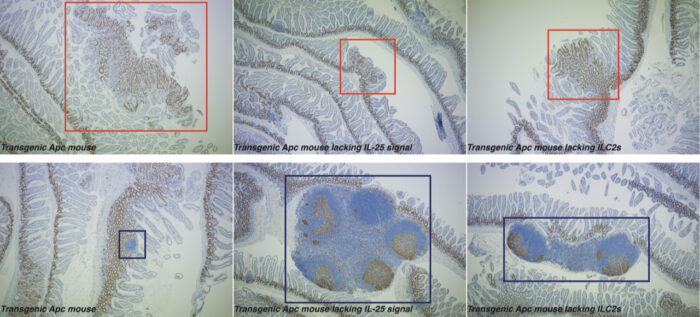
Colorectal cancer (CRC) is the second leading cause of cancer-related deaths worldwide. Current immunotherapy treatments, which rely upon the activation of the adaptive immune system to attack cancer cells, are largely ineffective in treating CRC. Cancer cells survive by successfully inhibiting the activity of the immune system, and can even manipulate immune cells so they promote cancerous growth. Andrew McKenzie’s group in the LMB’s PNAC Division have shown that by blocking the action of an intestinal immune cell, innate lymphoid cell 2 (ILC2), they can incite a protective immune response to better fight colorectal cancer.
CRC has several variants, but ~80% of them are linked to mutations in the adenomatous polyposis coli (APC) tumour suppressor gene. To replicate these conditions, the group used a pre-clinical transgenic Apc mouse model which mirrors the early events in human CRC. They found that when they blocked interleukin-25 (IL-25), an important signalling protein in the intestine, from reaching nearby ILC2s, the immune response became more anti-cancerous. This led to the mice developing fewer tumours and doubled their lifespan.
ILC2s usually help promote tissue repair and immunity to parasites within the intestines. Eric Jou, an MB/PhD student in Andrew’s group, noticed that eliminating ILC2s in the intestinal wall boosted the anti-cancer activity of other immune cells overall. Further investigation revealed that ILC2s were encouraging myeloid suppressor cells to subdue the anti-cancer immune response. By removing ILC2s from the intestine, or even simply limiting their function, myeloid cells and lymphocytes were better able to promote an anti-cancer response.
Further tests on human CRC tissue, provided by Simon Buczacki at Addenbrooke’s Hospital, Cambridge (now at the Nuffield Department of Surgical Sciences, University of Oxford), revealed a similar relationship between IL-25 and ILC2, with a correlation between higher tumour-related expression of the IL25 gene and reduced CRC patient survival. Analysis of patient samples showed that compared to healthy intestinal tissue, cancerous samples had increased infiltration of ILC2s expressing the receptor which allows them to respond to IL-25. The IL-25 receptor-blocking antibody used in this study was developed in collaboration with LifeArc, the medical research charity which provides technology transfer support to the MRC. Interestingly, this antibody also cross-reacts with the human IL-25 signalling pathway and hence, may prove beneficial to future human studies.
This study highlights the importance of ILC2s and other ILCs in controlling a balanced response within our immune system, particularly when responding to cancer. Better understanding of the mechanisms regulating this immune homeostasis could lead to clinical improvements for disease treatment and prevention.
This work was funded by UKRI MRC, Wellcome Trust, International Journal of Experimental Pathology, University of Cambridge, Marie Skłodowska-Curie Actions and Rosetrees Trust
Further references
An innate IL-25-ILC2-MDSC axis creates a cancer-permissive microenvironment for Apc mutation-driven intestinal tumorigenesis. Jou, E., Rodriguez-Rodrigues, N., Ferreira, ACF., Jolin, HE., Clark, PA., Sawmynaden, K., Ko, M., Murphy, JE., Mannion, J., Ward, C., Matthews, DJ., Buczacki, SJA., McKenzie, ANJ. Science Immunology
Andrew’s group page
Simon Buczacki’s page
Previous Insight on Research
How the fate of immune cell precursors is decided
Major breakthrough identifies cause and treatment of fatal autoimmune disease