Porous capillaries in the peripheral region of synovial joints provide access to circulating immune complexes – a blood-joint barrier of macrophages and neurons works to defend joint tissue
In many conditions, including autoimmune and infectious diseases, people commonly report pain or inflammation in joints, even though they may be physically distant from the site of the initial affliction. This often occurs in synovial joints – freely mobile joints that allow for a wide range of motion – which effectively act as a ‘barometer’ of systemic inflammation. This pain or inflammation is prompted by circulating immune complexes interacting with the vascular network and abundant pain fibres found in the synovial membrane. However, it has been unclear how these immune complexes access these synovial joints and trigger inflammation. Tetsuo Hasegawa and Menna Clatworthy, in the University of Cambridge’s Molecular Immunity Unit housed at the LMB, have imaged a mouse synovial joint in 3D to identify the mechanism behind this.
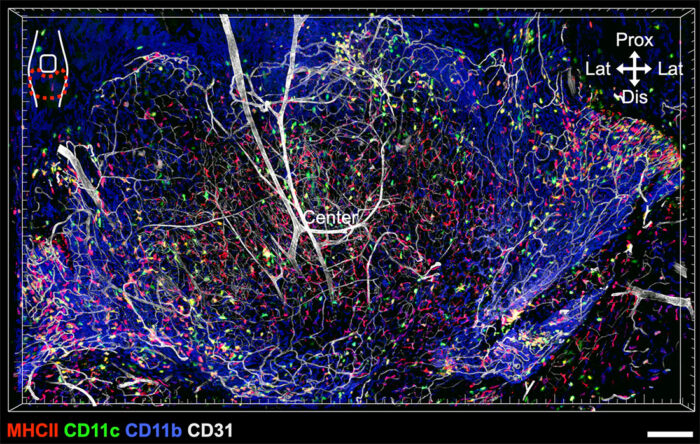
First, the pair utilised a stereoscopic microscope to dissect the entire synovium (the connective tissue which lines joints) in a mouse knee joint and applied staining to the tissue to image it. This is very technically challenging, as a healthy mouse synovium is transparent and extremely thin and therefore easy to damage during removal. The successful dissection of the intact synovium allowed Tetsuo and Menna to assess the spatial arrangement of the synovial immune cells in context alongside supportive neurovascular structures. This gave unprecedented insights compared to previous investigative efforts which examined sections of a divided joint.
Following this, they utilised flow cytometry, RNA-sequencing, and ex vivo culture of the entire synovia and neighbouring sensory neurons known as dorsal root ganglia. This approach allowed them to visually map the immune cell and neuronal responses when they introduced fluorescently labelled immune complexes.
They found that fenestrated capillaries were specifically located at the outer edge of the synovial tissue lining, next to where it inserts into bone. These capillaries possess small pores allowing circulating stimuli to access the joint. They also identified three distinct macrophage subsets at the periphery of the synovium, each of which performed distinct actions in response to challenges from systemic immune complexes. This included producing chemokines and cytokines to direct immune cells to the required site and forming clusters to encase capillaries. This region also included nociceptor neurons, which sense potentially harmful stimuli and send signals to the brain to alert it of potential injury. Ultimately, this underlines how a system of neuroimmune crosstalk at the blood-joint barrier acts as sentinels to defend vulnerable areas around fenestrated capillaries.
This work reveals a mechanism to explain the manifestations of infections in joints physically distanced from the centre of the body. Additionally, the newly identified bed of fenestrated capillaries at the synovia-bone interface, is the same site which forms abnormal layers of granulation tissue in rheumatoid arthritis. Therefore, this may explain why autoantibodies associated with rheumatoid arthritis activate macrophages to attack tissue specifically at this site for those with this condition.
This research does raise the question of why a blood-joint barrier has evolved to relay the presence of circulating stimuli by generating pain responses in the first place. Tetsuo and Menna posit that this warning system could exist to provide a selective advantage at both an individual and species level. Firstly, joint pain and inflammation might reduce mobility, thereby conserving energy for immune responses. Secondly, reduced mobility could help limit the transmission of infectious diseases to others. Similar theories have been explored to explain why some infection-associated cytokines are associated with increased levels of anxiety and reduced socialisation.
Though Tetsuo and Menna conducted imaging on human synovial tissue to confirm the presence of macrophages around capillaries, further work is needed to explore how this is disrupted in joint diseases like rheumatoid arthritis.
This work was funded by Human Frontier Science Program, Kennedy Trust, Wellcome, and Japan Society for the Promotion of Science KAKENHI.
Further references
Macrophages and nociceptor neurons form a sentinel unit around fenestrated capillaries to defend the synovium from circulating immune challenge. Hasegawa, T., Clatworthy, M., Lee, C., Hotchen, A. J., Fleming, A., Singh, R., Suzuki, K., Michisuke, Y., Watanabe, M., Birch, M., McCaskie, A., Lénárt, N., Krisztina, T., Denes, A., Liu, Z., Ginhoux, F. and Richoz, N. Nature Immunology
Menna’s group page – Cambridge Immunology Network
Molecular Immunity Unit (housed at the LMB)
Previous Insight on Research articles
How immune responses differ between asymptomatic cases and people with severe COVID-19
The brain’s immune defence comes from your gut
Balancing appropriate immune response in the gut