Amyloid filaments of tau protein found tethered to the membranes of brain extracellular vesicles
The abnormal assembly of tau protein into amyloid filaments in brain cells plays a central role in multiple neurodegenerative diseases, including Alzheimer’s disease. Targeting tau assembly for diagnostic and therapeutic benefit is reliant upon a greater understanding of the structures and behaviour of tau in disease. Now, Benjamin Ryskeldi-Falcon’s group in the LMB’s Neurobiology Division, in collaboration with Karen Duff’s group at the UK Dementia Research Institute, have used a combination of mass spectrometry proteomics and electron cryo-microscopy (cryo-EM) to investigate an extracellular form of tau pathology associated with extracellular vesicles.
Extracellular vesicles are a diverse group of membrane particles used by cells to convey complex signals. Previous studies had spotted assembled tau associated with these vesicles in the central nervous system of individuals with Alzheimer’s disease. This had been linked to the clearance of assembled tau from neurons, as well as the transfer of assembled tau between neurons, in cell and animal models. This may relate to the progression of tau pathology among connected brain regions over the course of disease.
The sensitive detection of tau-associated vesicles in extracellular fluids offers an attractive diagnostic strategy for Alzheimer’s disease and other tauopathies. Targeting the association of assembled tau with extracellular vesicles may also represent a therapeutic strategy to limit the progression of tau pathology. However, the structural and molecular characteristics of both the extracellular vesicles and the associated tau species were not known. In addition, it was not known how assembled tau associated with extracellular vesicles.
To begin, co-first author Stephanie Fowler, then a postdoc in the Duff group, fractionated extracellular vesicles from post-mortem brain tissue donated by individuals with Alzheimer’s disease. She used quantitative mass spectrometry and a panel of antibodies to profile the protein composition of the vesicles. This showed that the vesicles associated with assembled tau were enriched in endolysosomal proteins. She also found that most of the assembled tau was truncated either side of the region that forms the ordered core of tau filaments.
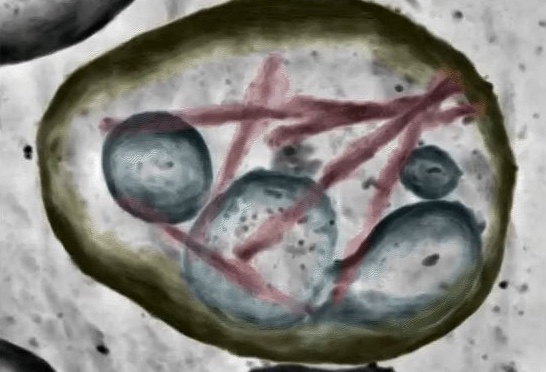
Co-first author Tiana Sophia Behr, who was a PhD student in the Ryskeldi-Falcon group, then used electron cryo-tomography (cryo-ET) to visualise the extracellular vesicles at molecular resolution under near-native conditions. By manually inspecting thousands of vesicles, she identified those that contained tau filaments. Using sub-tomogram averaging, as well as single-particle cryo-EM of filaments extracted from vesicles, she confirmed they were the paired-helical and straight tau filament structures of Alzheimer’s disease. The single-particle cryo-EM also showed that the filaments contained additional molecules coordinated to solvent exposed, positively charged amino acid side chains, compared to filaments found in brain cells. Unexpectedly, the cryo-ET revealed that the ends of tau filaments were tethered to the limiting membranes of the vesicles by flexible densities. This is the first report of membrane-tethering of amyloid filaments and highlights the power of cryo-ET to reveal novel molecular pathology of neurodegenerative diseases.
Together, these results provide structural and molecular insights into tau pathology associated with extracellular vesicles. They will guide strategies to target extracellular tau pathology for the diagnosis and treatment of Alzheimer’s disease and associated neurodegenerative diseases.
This work was enabled by brain donations from patients. It was funded by UKRI MRC, the UK Dementia Research Institute, the National Institutes of Health, the Alzheimer’s Association, the Alzheimer’s Society and Alzheimer’s Research UK.
Further references
Tau filaments are tethered within brain extracellular vesicles in Alzheimer’s disease. Fowler, S.L., Behr, T.S., Turkes, E., O’Brien, D.P., Cauhy, P.M., Rawlinson, I., Edmonds, M., Foiani, M.S., Schaler, A., Crowley, G., Bez, S., Ficulle, E., Tsefou, E., Fischer, R., Geary, B., Gaur, P., Miller, C., D’Acunzo, P., Levy, E., Duff, K.E., Ryskeldi-Falcon, B. Nature Neuroscience
Benjamin’s group page
Previous Insight on Research articles
First atomic structures of Tau filaments from Alzheimer’s disease brain