The signalling molecule tyramine is required for activation of molecular defence pathways in response to stress and promotes decisions that increase survival chances
When a C. elegans nematode worm senses a stressful environment, responses within the nervous system lead to activation of molecular defence mechanisms in other tissues, but how exactly this is signalled has been an outstanding question. Rebecca Taylor’s group, in the LMB’s Neurobiology Division, has identified a signalling molecule essential for communicating this message throughout the worm, triggering global defence responses as well as changes in feeding behaviour and reproduction.
Rebecca had previously discovered that expression of a protein, XBP-1s, in neurons could activate a stress response programme in the intestine of worms, increasing their longevity and making them more resistant to environmental stress. More recently, her group found that neuronal XBP-1s expression led to more active intestinal lysosomes, which can act as a waste disposal system in our cells, providing a possible mechanism for increased longevity. Her group, in collaboration with William Schafer’s group, also in the Neurobiology Division, and Olivia Casanueva’s group at the Babraham Institute, now wanted to identify the signal that connects XBP-1s expression in neurons with effects elsewhere in the worm.
Using worms that constantly express XBP-1s in neurons, Neşem Özbey, a PhD student in Rebecca’s group, found that a small molecule called tyramine was needed to allow activation of a particular stress response called the endoplasmic reticulum unfolded protein response (UPRER) in other tissues. In fact, XBP-1s expression in just the two pairs of neurons in the worm nervous system that produce tyramine was enough to activate this stress defence, and they found that these neurons naturally produced XBP-1s when the worm was starved, suggesting that this might be a part of the animal’s response to low nutrient conditions.
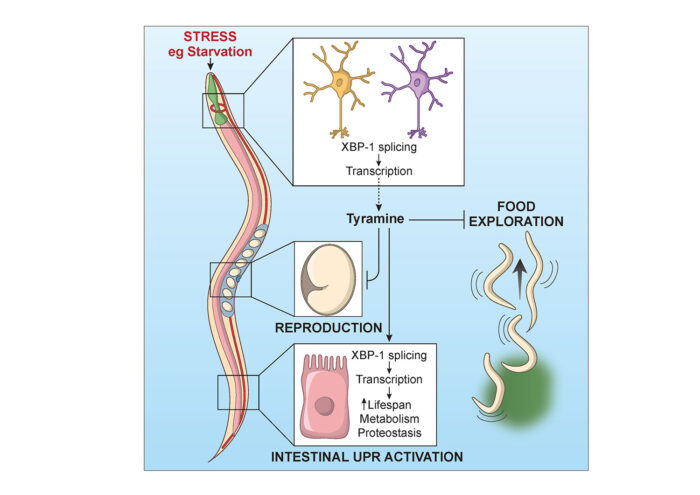
The team also found that this system affected behaviour of the worms, with XBP-1s expression and tyramine production making the animals reluctant to leave a source of food to explore, and reducing the number of offspring they produce. This means that detection of a stressful environment, such as one with little food, will activate molecular defences and promote decisions that increase the chances of survival for the animal and its offspring, with tyramine playing a key role in this process.
The UPRER is also an important defence pathway in human cells and may protect against neurodegenerative disease development. However, this stress response seems to become less active in older cells. Understanding how it is switched on in worms may help us to develop therapies that can do the same in human cells to slow the pace of ageing and the onset of age-related diseases, such as dementia, which are increasing problems in our society.
The work was funded by UKRI MRC.
Further references
Tyramine acts downstream of neuronal XBP-1s to coordinate inter-tissue UPRER activation and behaviour in C. elegans. Özbey, NP., Imanikia, S., Krueger, C., Hardege, I., Morud, J., Sheng, M., Schafer, WR., Casanueva, MO., Taylor, RC. Developmental Cell, 55, 1-17.
Rebecca’s group page
William’s group page
Olivia Casanueva’s group page
Previous Insight on Research