A joint project between the LMB and AstraZeneca reveals nearly 200 genes required for organisation of the cell’s internal space by the dynein motor
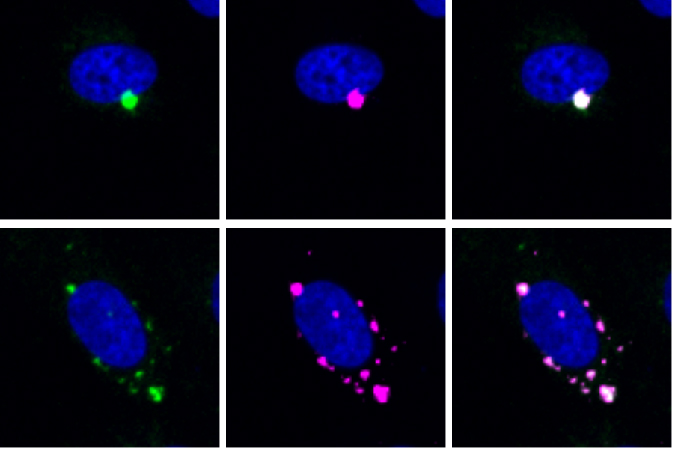
Chun Hao Wong from Simon Bullock’s group in the LMB’s Cell Biology Division has conducted a large-scale genetic screen with Douglas Ross-Thriepland and colleagues at AstraZeneca to reveal a large number of new factors required for organisation of cells by the microtubule motor dynein.
In order for cells to perform their elaborate functions, their internal parts – including organelles, vesicles and macromolecules – must be localised to the right place at the right time. This is achieved through the action of ‘microtubule motors’, proteins that associate with cellular ‘cargoes’ and deliver them to their destination by walking along microtubule tracks in the cytoplasm. Understanding this process has clear disease relevance – defects in this system in neurons can lead to neurogenerative disorders, and several pathogenic viruses exploit these transport routes to establish an infection.
A key player in the cellular transport system is the dynein motor, which is responsible for almost all cargo trafficking from the periphery of the cell to its interior. Our understanding of how dynein function is orchestrated has largely been restricted to studies of a handful of proteins that have typically involved working with them outside the cell. It was therefore not clear how these proteins operate in their natural environment or if additional proteins are required for the activity of the motor.
To address this issue, Chun Hao Wong, a post-doc in Simon Bullock’s group worked with Douglas Ross-Thriepland and colleagues at AstraZeneca to identify new genes that are needed for dynein’s functions in cultured human cells. Utilising AstraZeneca’s advanced automation platforms and expertise in genome editing at scale, Wong knocked out the function of the vast majority of genes in the human genome and examined the consequences for localisation of two of dynein’s cargoes by high-throughput microscopy.
To facilitate the identification of the important genes, the team took the ambitious step of targeting a separate gene in each well of a large set of tissue culture plates. From images of over 8 million cells, Wong was able to identify and validate nearly 200 genes that affect dynein-based transport, including many that were important for only a subset of the motor’s cargoes.
Using statistical approaches to group together ‘fingerprints’ derived from the distribution of several molecules in edited cells, the team was able to identify several genes that are strong candidates to affect dynein function directly. Follow up studies of one of these factors, the RNA-binding protein SUGP1, provided a proof-of-principle of how the screen can lead to new mechanistic insights. This aspect of the study involved teaming up with two experts in computational analysis of cellular RNA content: Steven Wingett from the LMB and Matthew Taliaferro from the University of Colorado.
The study is significant as it provides a rich source of new hypotheses for investigating dynein-based transport, as well as other aspects of cellular organisation that were captured in the microscopy images. To maximise the output from the project, the images from the screen are available to other researchers through AstraZeneca’s Open Innovation platform. Moreover, once their mode of action is elucidated, hits from the dynein screen might offer new routes for alleviating neurodegeneration and viral infection by manipulating microtubule-based transport.
This project was supported through a research collaboration between AstraZeneca UK Limited and the Medical Research Council (BSF31). This work was also funded by UKRI MRC and NIH.
Further references
Genome-scale requirements for dynein-based transport revealed by a high-content arrayed CRISPR screen. Wong C.H., Wingett S.W., Qian C., Hunter M., Taliaferro J.M., Ross-Thriepland D. and Bullock, S.L. Journal of Cell Biology
Simon’s group page
AstraZeneca
Blue Sky Collaboration
AstraZeneca Open Innovation platform
Matthew Taliaferro’s group page
Previous Insight on Research articles
Repeating peptides are obstacles to neuronal transport in motor neurone disease
Reversing a decline in cellular transport in ageing nerve cells