High-speed droplet vitrification eliminates protein damage at the air–water interface, unlocking more reliable structure determination in cryo-EM
Electron cryomicroscopy (cryo-EM) has revolutionised structural biology by allowing scientists to visualise proteins and complexes at atomic resolution. To do this, samples are frozen very quickly in a thin layer of water, then scanned with an electron beam. However, a persistent challenge has limited its potential: during specimen preparation, proteins diffuse to the air-water interface before they freeze, where they often adhere and become damaged. This damage compromises the integrity of samples and forces researchers to discard most of their collected data – in fact, more than 99% of images are often unusable.
Now, Chris Russo’s group, in the LMB’s Structural Studies Division, in collaboration with Kasim Sader, Associate Director of Cryo-EM in BioPharmaceuticals Research & Development (R&D) at AstraZeneca, have developed a new method that freezes proteins so fast that it prevents them from ever reaching the air–water interface. Chris and Anastasiia Gusach, a postdoc in Chris’s group, did this by spraying microscopic droplets of protein solution at very high speed (approaching 100 m/s – about the same speed as a jumbo jet) onto cryogenically cooled grids coated with liquid ethane. The droplets flatten and freeze in in under 10 microseconds, locking the proteins in place before they can diffuse across the water layer.

This breakthrough was made possible by a droplet sprayer custom-built by the LMB’s Electronics and Mechanical Workshops, capable of delivering droplets between 5 and 30 micrometres with precise control. Upon impact with the ethane-coated grid, the droplets flatten and freeze almost instantly. Slow motion videos taken with high-speed cameras confirmed that the spreading and freezing occurred in microseconds, effectively interrupting protein diffusion and eliminating contact with the damaging interface.
To validate the method, the team compared traditional blotting-based plunge freezing with their new approach using tomographic reconstructions. Conventional methods showed protein accumulation at the interface, while the sprayed specimens maintained uniform concentrations matching the original solution. Further cryo-EM imaging of the protein apoferritin yielded a 2.7 Å resolution reconstruction from over 100,000 particles, with no evidence of structural degradation from the spraying process.
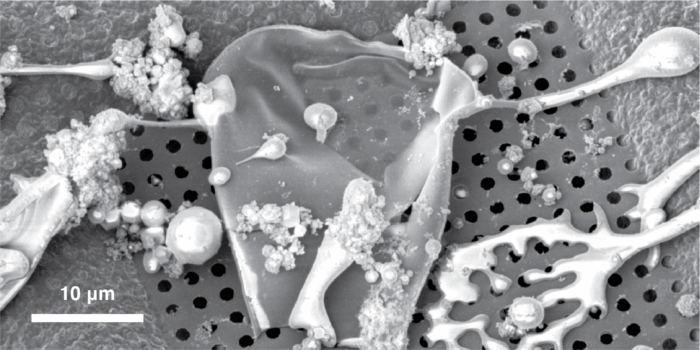
While the method does not yet solve all challenges, such as controlling ice thickness and particle orientation, it marks a significant step toward automated, reproducible cryo-EM specimen preparation. By eliminating the need for surfactants or support films and preserving fragile protein interactions, this technique opens new possibilities for studying complex biological assemblies and disease-related proteins.
This work was funded by UKRI MRC and further supported by the BlueSky collaboration between AstraZeneca UK Limited and the Medical Research Council (BSF2-08), which supports pre-clinical research projects with the aim to improve understanding of fundamental biology and disease.
Further references
Outrunning protein diffusion to the air-water interface in cryoEM. Gusach, A., Sader, K. and Russo, C.J. PNAS
Chris’s group page
Blue Sky Collaboration
Previous Insight on Research articles
A colder frontier: cryo-EM at liquid helium temperatures
Low energy cryo-EM to widen accessibility for fast and accurate structure determination
Scaling-up grid manufacture to solve bottleneck in cryo-EM
Freezing molecules completely still for cryo-EM