 Rebecca Voorhees and Manu Hegde, from the LMB’s Cell Biology Division, have used electron cryo-microscopy (cryo-EM) to determine how a channel that is essential for protein transport is opened. This channel, known as Sec61 in mammals, is needed for secretion of proteins from the cell and insertion of proteins into the membrane. Secretory and membrane proteins represent nearly one-third of all proteins in any organism, from bacteria to humans, and perform many diverse functions including sensing the environment, communication with other cells, and metabolite transport into and out of the cell. Understanding how this important class of proteins are transported across or inserted into an otherwise impermeable membrane has been a long-standing goal in biology.
Rebecca Voorhees and Manu Hegde, from the LMB’s Cell Biology Division, have used electron cryo-microscopy (cryo-EM) to determine how a channel that is essential for protein transport is opened. This channel, known as Sec61 in mammals, is needed for secretion of proteins from the cell and insertion of proteins into the membrane. Secretory and membrane proteins represent nearly one-third of all proteins in any organism, from bacteria to humans, and perform many diverse functions including sensing the environment, communication with other cells, and metabolite transport into and out of the cell. Understanding how this important class of proteins are transported across or inserted into an otherwise impermeable membrane has been a long-standing goal in biology.
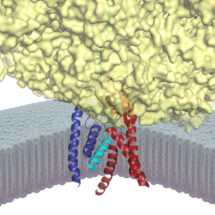
The Sec61 channel performs these tasks by providing conduits across and into the membrane. These conduits are normally closed, and only open when Sec61 is engaged by an appropriate signal peptide in a nascent secretory or membrane protein. Despite many advances in studying the architecture and function of Sec61, the molecular basis for how a signal peptide triggers the channel to open was not understood.
To address this problem, Rebecca and Manu devised a way to trap and purify the Sec61 channel at the precise stage when it has engaged the signal of a nascent secretory protein. This sample was then analyzed by cryo-EM and its molecular structure was reconstructed. The new structure provides the first high resolution view of an open and functioning protein conducting channel. Earlier work, including that from the LMB, had observed Sec61 in different closed states. By comparing the new Sec61 structure to these previous structures, they have predicted a two-step mechanism for how the channel might be selectively gated by hydrophobic signals. The first step involves the “priming” of Sec61 when it binds to a ribosome, the protein synthesis machine of cells. The primed Sec61, while not open, is partially destabilized. If the bound ribosome is synthesizing a protein containing a signal peptide, the signal can engage Sec61 at the precise position that was partially weakened by priming. The signal then embeds into the wall of Sec61, thereby expanding its diameter slightly to open a channel across the membrane. The nascent protein can now be transported through this channel, or in the case of a membrane protein, enter the membrane at the place where the signal is lodged. Such a two-step gating mechanism is essential for ensuring that only bona fide secretory and membrane proteins gain access to the correct membrane compartments.
Defects in secreted and membrane protein maturation and trafficking underlie numerous protein misfolding diseases such as cystic fibrosis and many types of neurodegeneration. The diverse and essential roles of these proteins, as well as the consequences of their aberrant trafficking, underscores the physiological importance of understanding how secreted and integral membrane proteins are properly recognized, targeted, and inserted in the lipid bilayer. The structure of Sec61 in this intermediate state of insertion, illustrates the detailed molecular pathway these proteins use to reach their final destination in the membrane.
This work was funded by the MRC and a Wellcome Trust postdoctoral fellowship.
Further references:
Paper in Science
Manu’s Group Page
Insight on Research – Cryo-EM reveals mammalian protein export machinery