Identification of the elusive proteins that act as the molecular connection between a mechanical stimulus, such as a sound or touch, and a nerve firing
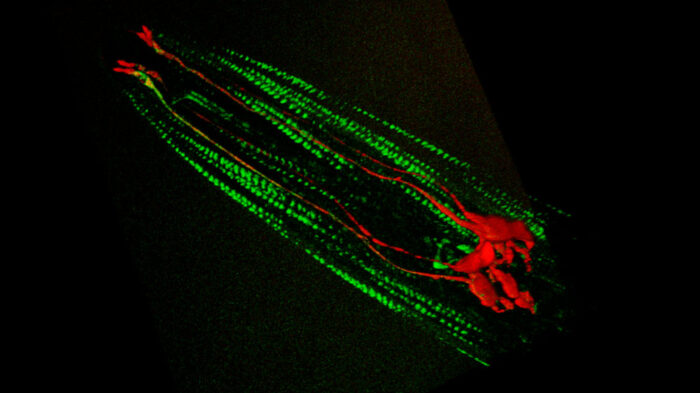
Hearing and touch depend on the ability of sensory neurons to be activated by a force, such as pressure or vibration from sound, and then pass information on to the brain. William Schafer’s group, in the LMB’s Neurobiology Division, has, for the first time, identified some of the key proteins that make this possible. Hearing is one of the least understood of our five senses. These proteins might be the missing pieces that solve the puzzle of how hearing works at the basic molecular level.
This process, known as mechanotransduction, involves a sensory neuron responding to a received mechanical force by opening specialised ion channels so that the neuron then fires an electrical signal. Although many of these mechanotransduction ion channels are known, the way that they are controlled by force has not been well understood.
One model of how a force might lead to opening of a mechanotransduction channel suggests the existence of an elastic filament, known as a gating spring, that tethers the channel to the cell’s internal cytoskeleton. This would allow external forces to be connected through to the channel via movement of the cytoskeleton. However, the identity of the gating spring had been elusive.
Using yeast 2-hybrid screening and mass spectrometry, Yiquan Tang, a postdoctoral researcher in William Schafer’s group, identified two proteins necessary for the function of mechanotransduction channels in C. elegans worms: CALM-1 and UNC-44. Collaborating with Hang Lu’s group at Georgia Tech and Siva Vanapalli’s group at Texas Tech University, two mechanical engineering groups with expertise in microfluidics, the team were able to study the roles of these proteins in mechanosensation by measuring responses to applied mechanical forces and forces generated by the muscles of mutant and normal worms.
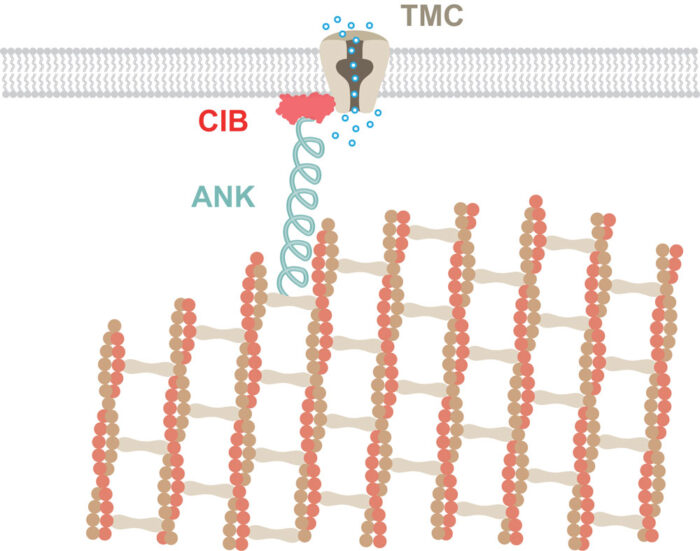
UNC-44 is the worm version of a protein family called ankyrins. Ankyrins are elastic filamentous proteins that interact with the cytoskeleton and therefore show the expected properties of a gating spring for mechanotransduction. Yiquan found that UNC-44 interacts with CALM-1 and that CALM-1 binds to mechanotransduction channels. This suggests that CALM-1 acts as an adaptor to link the gating spring ankyrin to the channel, completing the chain link from cytoskeleton to ion channel. Interestingly, this same complex is used to detect force in muscle cells, showing how the same molecular machines can be reused in seemingly unrelated biological processes.
Importantly, CALM-1 is the worm version of the human CIB2 protein, mutations in which have been associated with deafness. This suggests that this complex might be conserved and that ankyrin might similarly act as the gating spring to allow hearing and touch sensation in humans. Understanding how these proteins work together to detect sound vibrations and how they might stop working in disease could help us understand hearing loss better and could potentially lead to treatment.
The work was funded by UKRI MRC, the Wellcome Trust, NIH, NASA, and the Cancer Prevention and Research Institute of Texas.
Further references
Ankyrins are intracellular tethers for TMC mechanotransduction channels. Tang, YQ., Lee, SA., Vanapalli, SA., Lu, H., Schafer, WR. Neuron 107: 1-14.