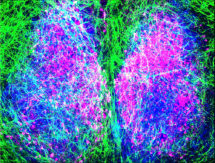
The suprachiasmatic nucleus of the hypothalamus (SCN) is our principal “body clock”, controlling our daily (circadian) rhythms of physiology and behaviour that adapt us to the 24-hour cycle of day and night. It ensures that numerous other local tissue clocks distributed across the body are in tune with each other and with the external light-dark cycle. It is generally assumed that the SCN achieves its circadian time-keeping role thanks to the highly specialised functions performed by its neurons. However, neurons are not the only cell-type present in the brain, and in the SCN almost half of the cells are astrocytes. These star-shaped cells are typically seen as sustaining and nourishing neurons, and elsewhere in the brain they are generally believed not to take an active part in information processing. Research lead by Marco Brancaccio, in Michael Hastings’s group in the LMB’s Neurobiology Division, has recently cast an entirely new light on the properties and activities of the astrocytes of the SCN and has shown that they play a pivotal role in the SCN clockwork.
The group have discovered that SCN astrocytes, unlike SCN neurons which are active in the day, are active during the night and that the simultaneous activation of these astrocytes is fundamental in controlling the activity cycle of SCN neurons. Nocturnal astrocytic activity supresses the neurons at night, and daily release of this inhibition synchronises the SCN neuronal network. The group also identified the chemical transmitter released by astrocytes causing this inhibition: glutamate, which is released at night into the extracellular space between astrocytes and neurons. They also revealed the neuronal receptors at which glutamate acts (NMDAR2C) to connect these two cell types in the SCN. This led to the discovery of a specific microcircuit in the dorsal part of the SCN, an area known to be strongly rhythmic and responsible for circadian pace-making.
These unprecedented findings were corroborated by in vivo experiments in mice, demonstrating that not only neurons, but also astrocytes of the SCN can impose their pace-making properties on the rest of the animal. Together, this work shows that there are two arms to the circadian master clock in the SCN: neurons and astrocytes, and they are both required to encode information controlling the mammalian body clock, through their mutually supportive, but temporally segregated cellular activities.
In recent years it has become abundantly clear that disturbances of the circadian body clock can have pronounced effects on health and well-being, as in rotational shift-work, dementia and sleep/ psychiatric disorders. This study may provide new opportunities to control the clock under circumstances where it goes wrong and reveal new opportunities for their potential therapy.
The work was funded by the MRC.