Cryo-EM study reveals structures of amyloid-beta 42 filaments; a key factor behind the pathogenesis of Alzheimer’s disease
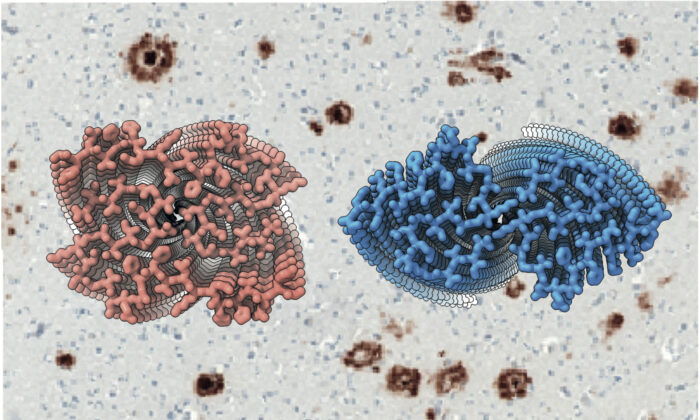
Alzheimer’s disease is defined by the simultaneous presence of two different filamentous amyloid inclusions in the brain: abundant extracellular plaques of amyloid-beta (Aβ) and intraneuronal neurofibrillary tangles of tau. Several lines of evidence have suggested that the formation of Aβ deposits drives the pathogenesis of Alzheimer’s disease. Even though Aβ peptides vary in size, those ending at residue 42 are the most abundant. Smaller numbers of Aβ42 deposits form also with age and can be associated with other neurodegenerative conditions. To better understand Alzheimer’s disease and these conditions, one needs to know the structures of Aβ filaments. Now, a collaborative study between the groups of Bernardino Ghetti from Indiana University and Benjamin Ryskeldi-Falcon, Sjors Scheres and Michel Goedert, from the LMB, has led to the determination of the structures of Aβ42 filaments.
Lead authors Yang Yang, Diana Arseni and Wenjuan Zhang used electron cryo-microscopy (cryo-EM) to determine the first high-resolution structures of Aβ42 filaments from the brains of five individuals with Alzheimer’s disease (three sporadic and two inherited) and five individuals with other neurodegenerative conditions (aging-related tau astrogliopathy, Parkinson’s disease-dementia, dementia with Lewy bodies, frontotemporal dementia and pathological aging). The new structures differ from those previously determined using in vitro assembled Aβ42.
The team discovered the presence of two structurally related S-shaped protofilament folds, which give rise to two types of Aβ42 filaments, each consisting of two identical protofilaments. Type I filaments were most abundant in sporadic Alzheimer’s disease and type II filaments in inherited Alzheimer’s disease and other conditions. The ordered core of the type I protofilament, which was resolved to 2.5 Å, extends from G9 to A42 and comprises five β-strands, whereas that of the type II protofilament, which was resolved to 2.8 Å, extends from V12 to A42 and comprises four β-strands.
In AppNL-F knock-in mice, which develop abundant deposits of wild-type human Aβ42, type II filaments were present. This is the first experimental system with amyloid filament structures like those from the human brain.
This study significantly advances our understanding of the pathogenesis of Alzheimer’s disease, the most common cause of dementia. It opens the way to the development of imaging agents with increased sensitivity and specificity. It may now also be possible to design compounds that inhibit Aβ42 assembly, which could be a viable therapeutic strategy for Alzheimer’s disease.
This work was funded by UKRI MRC, Alzheimer’s Research UK, the Rainwater Charitable Foundation, the US National Institutes of Health, Indiana University School of Medicine, the Safra Foundation and the Rossy Foundation.
Further references
Cryo-EM structures of amyloid-β 42 filaments from human brain. Yang, Y., Arseni, D., Zhang, W., Huang, M., Lövestam, S., Schweighauser, M., Kotecha, A., Murzin, AG., Peak-Chew, SY., Macdonald, J., Lavenir, I., Garringer, HJ., Gelpi, E., Newell, KL., Kovacs, GG., Vidal, R., Ghetti, B., Ryskeldi-Falcon, B., Scheres SHW., Goedert, M. Science
Michel’s group page
Sjors’ group page
Benjamin’s group page
Bernardino Ghetti’s page
Science Perspective: A molecular view of human amyloid-β folds
Previous Insight on Research articles
Classification of human tauopathies based on tau filament folds
First structures of α-synuclein filaments from human brain
A novel tau fold in the neurodegenerative disease corticobasal degeneration