Human choroid plexus organoids produce cerebrospinal fluid and form a highly selective barrier that could be used to predict the permeability of novel drugs
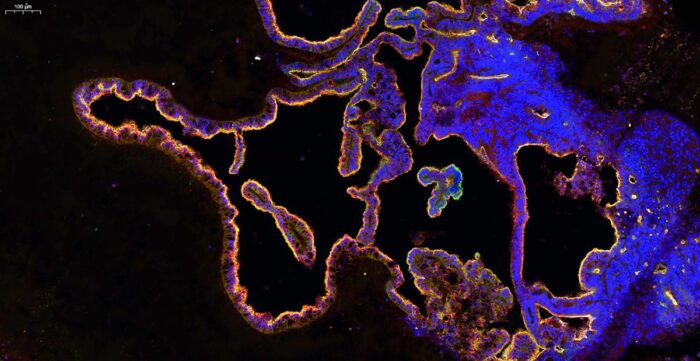
The human brain is bathed in a supportive fluid called the cerebrospinal fluid (CSF) that provides various nutrients and is required for proper brain function. Current understanding of the composition of human CSF, and how it is made, is limited due to a lack of experimental access. Madeline Lancaster’s group, in the LMB’s Cell Biology Division, have now developed a new brain organoid that produces CSF and has the potential to predict whether drugs can access the brain.
CSF is produced and secreted by a tissue found deep within the brain called the choroid plexus (ChP). The ChP also filters blood, acting as a barrier to most substances found in the blood, but selectively permitting access of some small molecules. To study the development and function of the human ChP, including how CSF is made, Madeline’s group developed a new organoid model of this tissue.
What are organoids?
Organoids are collections of organ-specific cell types that are produced from stem cells and can be studied as miniature, simple versions of organs. Organoids display similar organisation of cell-types to the organ they model and can perform some specific functions.
Scientists produce organoids by directing stem cells towards particular cell-types through the use of signalling molecules, similarly to how the tissues would arise during development.
To develop a “human CSF in a dish” model, Laura Pellegrini, from Madeline’s group, established a protocol to produce ChP organoids from human stem cells based on the group’s method for producing cerebral organoids. These organoids display key features of human ChP and develop CSF-filled compartments that are isolated from the surrounding culture media that the organoids are grown in.
The team found that this fluid contained known CSF biomarkers and they were able to observe changes in secretion of CSF components over time as well as the distinct cell-types contributing to these dynamic changes of CSF composition. Importantly, they discovered a previously unidentified cell type in the ChP: myoepithelial cells. These cells could be important in the generation of mechanical forces involved in CSF secretion.
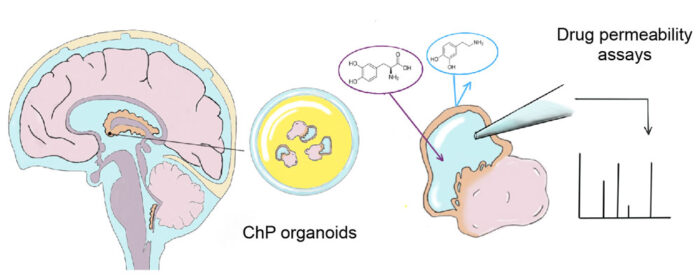
The ChP organoids were also shown to form a tight barrier that exhibited the same selectivity for small molecules as is seen for the ChP in the brain, for example they prevented entry of the small molecule dopamine, but allowed transport of its precursor, L-Dopa. As well as showing the accuracy of the model to the tissue that it is representing, this also means that ChP organoids could have predictive potential for the permeability of novel drugs. To demonstrate this, the team investigated one drug that recently failed in phase 1 clinical trials, BIA-10-2474, and found that these organoids could have predicted that the drug would inappropriately accumulate and cause neurotoxicity.
The composition of the brain barrier is different between species, so development of a human-specific ChP model is important for its capacity to predict the permeability of novel drugs and reduce the number of new drugs that fail in phase 1 clinical trials. This model is also very important as a source of more authentic CSF that has been made specifically by ChP tissue. This will enable scientists to study the secretion of different factors and disease-related biomarkers whose functions are still not well understood.
The work was funded by UKRI MRC and the European Research Council.
Further references
Human CNS barrier-forming organoids with cerebrospinal fluid production. Pellegrini, L., Bonfio, C., Chadwick, J., Begum, F., Skehel, M., Lancaster, MA. Science (Epub ahead of print)
Madeline’s group page