Cell death in response to detection of lipopolysaccharide within the membrane of cytosolic bacteria is driven by assembly of a signalling platform on the bacterial surface
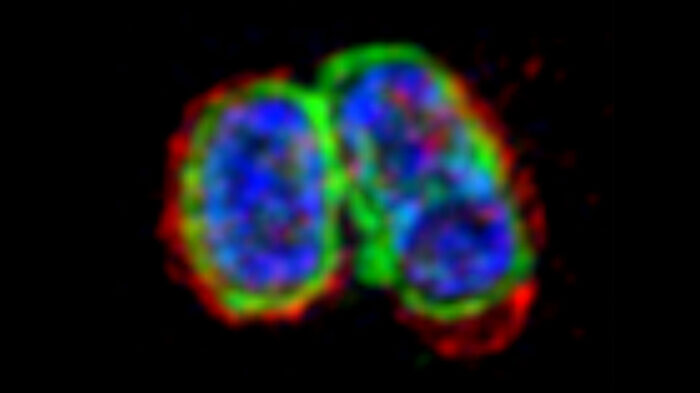
Septic shock is one of the most common causes of death in developed countries and is associated with a high rate of mortality, despite advances in critical care medicine. One trigger of septic shock is lipopolysaccharide (LPS), a component of the outer membrane of the group of bacteria known as Gram-negative bacteria. Although it is known that detection of LPS inside cells can lead to cell death, this LPS-sensing pathway is not well understood. Felix Randow’s group, in the LMB’s PNAC Division, has now shown how the surface of cell-invading bacteria is transformed into a signalling platform to activate this pathway.
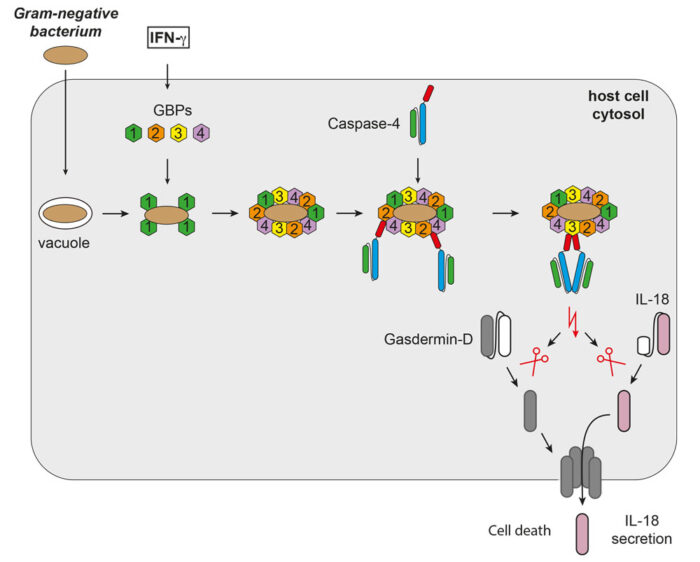
It has been known for some time that extracellular LPS is detected by the receptor TLR4, but a second LPS-sensing pathway that reacts to intracellular LPS was only discovered more recently. In this pathway, LPS activates the protease enzyme caspase-4, which then activates the pore-forming protein Gasdermin-D, leading to a form of cell death known as pyroptosis. However, previous studies of this pathway used soluble LPS, so it was not known how caspase-4 could become activated when LPS is embedded within a bacterial membrane.
To address this question, Michal Wandel, a researcher in Felix’s group, used super-resolution structured illumination microscopy to discover how a group of proteins called guanylate-binding proteins (GBPs) assemble on the surface of cytosol-invading bacteria to create a signalling platform that recruits and activates caspase-4. This signalling platform involved multiple different GBPs that acted sequentially with different functions: initiating platform assembly, recruiting caspase-4, and activating caspase-4.
Michal went on to show that this GBP-driven pathway was required for caspase-4 to activate the pore-forming Gasdermin-D and the proinflammatory cytokine interleukin-18 (IL-18) that is then secreted to alert nearby cells to the presence of cytosol-invading bacteria. Through collaboration with Matthias Zilbauer’s group at the University of Cambridge and John MacMicking’s group at Yale University the team were able to confirm their findings in human gut organoids and mice.
Although this work identifies the proteins that take part in this LPS-sensing pathway, structures are needed for a greater mechanistic understanding of this signalling platform. This would be of great benefit to help us understand how the platform assists in the activation of caspase-4. Future studies are also needed to determine whether similar pathways are used for bacteria that don’t have LPS in their outer membranes.
Sepsis arises from an over-activation of the immune response against bacterial toxins, including LPS. This finding that caspase-4 activation of pyroptotic cell death and IL-18 proinflammatory signalling is dependent on GBPs suggests that GBPs could be useful targets of pharmacological inhibitors to reduce the immune response and prevent septic shock.
The work was funded by UKRI MRC, the Wellcome Trust, the NIH National Institutes of Allergy and Infectious Diseases, and the Howard Hughes Medical Institute.
Further references
Guanylate-binding proteins convert cytosolic bacteria into caspase-4 signaling platforms. Wandel, MP., Kim, B-H., Park, E-S., Boyle, KB., Nayak, K., Lagrange, B., Herod, A., Henry, T., Zilbauer, M., Rohde, J., MacMicking, JD., Randow, F. Nature Immunology (Epub ahead of print)
Felix’s group page
John MacMicking’s page
Matthias Zilbauer’s page