The Drosophila larval brain is the most complex brain so far for which the map of connections between all neurons has been obtained
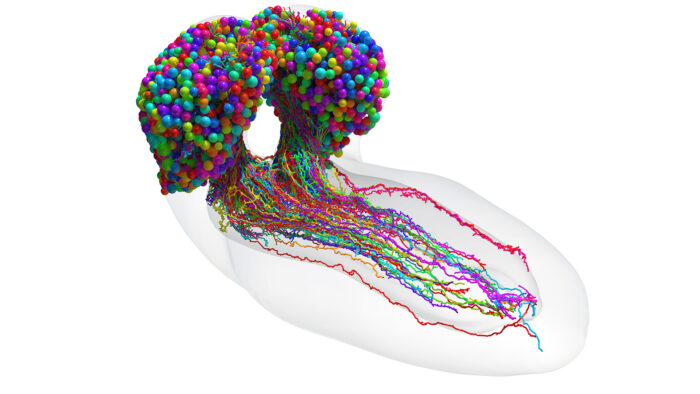
To understand fully how a brain generates behaviour, a synaptic-resolution map of the entire brain detailing all its neural connections – known as a connectome – is necessary. Previously, only three organisms (each with several-hundred neurons) have been mapped to show their complete connectomes: Caenorhabditis elegans, Ciona intestinalis larva, and Platynereis dumerili larva. For the larger brains of insects, fish and mammals, synapse-resolution circuity has been approached by mapping select regions in isolation. Now, for the first time, the synaptic-resolution connectome of an entire brain of an insect, the Drosophila melanogaster larva, comprising 3016 neurons and 548,000 synapses has been mapped using computer-assisted reconstruction from electron micrographs. This work was spearheaded by the groups of Marta Zlatic and Albert Cardona in the LMB’s Neurobiology Division, as well as members of their groups in the University of Cambridge’s Department of Zoology and Department of Physiology, Development, and Neuroscience respectively, in collaboration with Joshua T. Vogelstein at Johns Hopkins University.
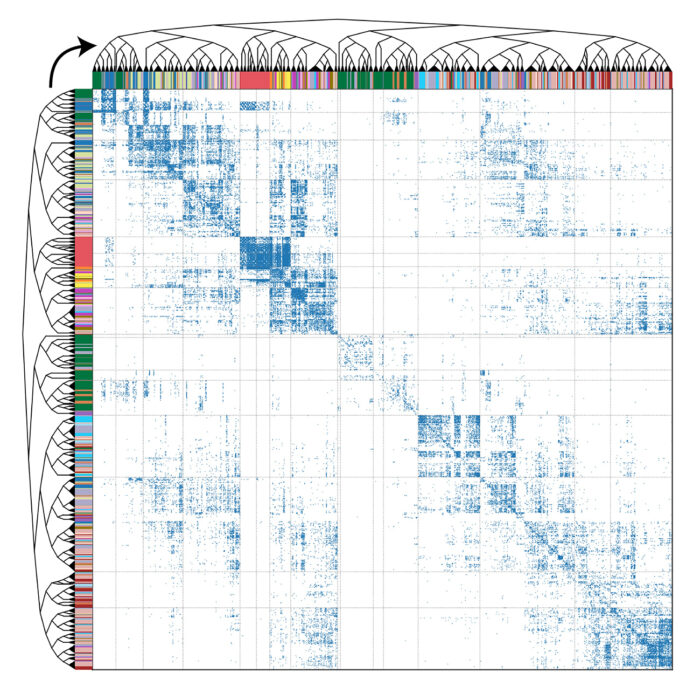
The brain of the Drosophila larva is capable of behaviour such as learning, value-computation and action-selection, and neural computations occur across spatially disparate, interconnected regions of the brain. Having mapped the synaptic-resolution connectome, the authors performed detailed analyses of the architecture of the circuits, including connection and neuron types, network hubs, and circuit motifs. They found that 73% of the brain’s in-out connecting hubs were postsynaptic to the learning centre, or presynaptic to the dopaminergic neurons which drive learning. Using graph spectral embedding, the group hierarchically clustered neurons by synaptic connectivity into 93 neuron types which were internally consistent with features such as morphology and function. A new algorithm was developed to track the brain’s signal propagation across polysynaptic pathways, and then feedforward (sensory to output) and feedback pathways, multisensory integration, and cross-hemisphere interactions were all analysed. The team identified extensive multisensory integration throughout the brain, with numerous interconnected pathways from sensory neurons to output neurons forming a distributed processing network.
The Drosophila brain’s architecture was highly recurrent, with 41% of neurons receiving long-range recurrent input. Recurrence was particularly elevated in regions involved in learning and action selection and dopaminergic neurons that drive learning were found to be among the most recurrent neurons in the brain. Additionally, the group identified that extensive communication across hemispheres was facilitated by in-out hubs comprised of contralateral neurons synapsed onto each other. Finally, the group also analysed interactions between the brain and nerve cord, finding that descending neurons targeted a minority of premotor elements which could be important in switching between locomotor states. A subset of descending neurons targeted low-order post-sensory interneurons, which likely modulate sensory processing.
This complete brain connectome is a landmark achievement, and will serve as the basis for future experimental and theoretical studies of neural circuits and brain function. The techniques and computational tools used in this approach can be further applied to analyse connectomes of more complicated animals. As connectomes from more organisms are mapped, researchers will be able to identify which circuit architectures are conserved and potentially optimal, as well as divergent architectures which may underlie behavioural differences between organisms.
Furthermore, it is notable that some of the features observed in the Drosophila brain, such as multilayer shortcuts and prominent nested recurrent loops, are found in innovative, artificial neural networks where they support task-dependent computations and compensate for a lack of network depth. Future analysis of the similarities and differences between connectome maps and artificial neural networks may help to illuminate brain computational principles and perhaps inspire new machine learning architectures.
This work was funded by HHMI Janelia Research Campus, UKRI MRC, Wellcome Trust, ERC, NSF, NIH, DARPA and the Air Force Research Laboratory.
Further references
The connectome of an insect brain. Winding, M., Pedigo, BD., Barnes, CL., Patsolic, HG., Park, Y., Kazimiers, T., Fushiki, A., Andrade, IV., Khandelwal, A., Valdes-Aleman, J., Li, F., Randel, N., Barsotti, E., Correia, A., Fetter, RD., Hartenstein, V., Priebe, CE., Vogelstein, JT., Cardona, A., Zlatic, M. Science
Marta’s group page
Albert’s group page
Joshua T. Vogelstein – Johns Hopkins University
UKRI news release
University of Cambridge news release