Components of shelterin, TPP1 and POT1, assist telomerase recruitment to telomeres and regulate the enzyme’s activity during DNA replication
The three-dimensional structures of the telomeric DNA-bound human telomerase in complex with recruitment factors, TPP1 and POT1, were determined by cryo-electron microscopy. The structures provide the molecular insights into telomerase recruitment to telomeres.
Telomeres – protective caps found at the end of eukaryotic chromosomes – play a vital role in maintaining genome stability. In mammals, telomeres are bound by a six-membered protein complex called shelterin. This complex is responsible for regulating the enzyme, telomerase, which expands telomeres during cell division. Telomerase helps to protect DNA in cells by compensating for losses during the inherently incomplete process of genome replication. A new study from Kelly Nguyen’s group in the LMB’s Structural Studies Division has provided structures, which reveal unprecedented insights into the molecular basis underlying how shelterin recruits telomerase, and activates telomerase for the processive extension of chromosome ends.
Group members, Zala Sekne and George Ghanim, solved the structure of telomerase with recruitment factors using cryo-electron microscopy (cryo-EM) – collecting over 50,000 images. RELION (a cryo-EM image processing programme developed by Sjors Scheres’ group) allowed the pair to resolve the structures of telomeric DNA bound to human telomerase in a complex with TPP1 and with TPP1-POT1 at 3.2 Å and 3.9 Å resolution, respectively.
The structures reveal how the TPP1 and POT1 heterodimer in shelterin interacts with telomerase in detail, including explaining how TPP1-POT1 stabilises DNA and facilitates telomerase engagement with telomeric DNA to extend the chromosomal ends more efficiently.
In addition, their work also uncovered a previously unknown DNA threading surface. This was confirmed by mutagenesis experiments in which the residues forming a DNA anchor site were revealed. The presence of a DNA anchor site had been proposed in previous studies yet its identity was unknown until now.
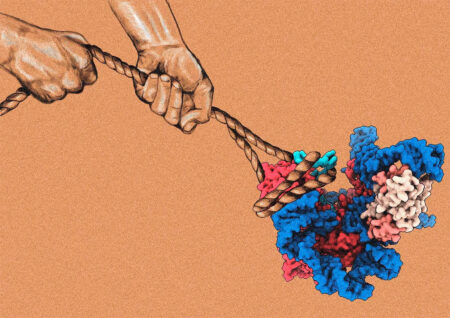
The discovery of these structures not only affirms postulations in previous studies but also sets the framework for future work on telomerase regulation. As telomerase regulates DNA elongation during cell division, the enzyme is an important drug target against cancers and ageing diseases. The deciphered structures open up various avenues for rational drug design when therapeutically targeting telomerase recruitment and activity.
This work was funded by UKRI MRC and the Jane Coffin Childs Fund.
Further references
Structural basis of human telomerase recruitment by TPP1-POT1. Sekne, Z., Ghanim, GE., van Roon, AMM., Nguyen, THD. Science.
Kelly’s group page
Previous Insights on Research
First atomic model of human telomerase constructed by electron cryo-microscopy