Structural study using electron cryo-microscopy provides the first detailed insight into molecular interactions between shelterin and nucleosomes at telomeres
Telomeres – the protective cap structures found at the ends of eukaryotic chromosomes – are essential for genome integrity as they safeguard chromosome ends from aberrant DNA repair and erosion. In mammals, telomeric cap structures consist of arrays of the six-membered protein complex called shelterin and nucleosomes. Nucleosomes are basic structural units of DNA packages in the eukaryotic nucleus, and are comprised of a section of DNA wrapped around a core of eight histone proteins. Most previous studies have individually focused on shelterin or nucleosomes, and as such there is limited understanding of how the two interact to form the protective capping structures.
Kelly Nguyen’s group, in the LMB’s Structural Studies Division, has worked with Carol Robinson’s group at the University of Oxford’s Department of Chemistry to investigate the interaction between the shelterin component TRF1 and telomeric nucleosome. To achieve this, they determined the electron cryo-microscopy (cryo-EM) structures of a telomeric nucleosome both bound and unbound to TRF1 to resolutions of 2.7 Å and 2.5 Å, respectively.
Analysis of the cryo-EM structures by Hongmiao Hu, Marike van Roon and George Ghanim, all members of Kelly’s group, found that upon binding to TRF1, the ends of the nucleosomal DNA are unwrapped from the core histone proteins and the DNA register is shifted. This is the first structural evidence for the nucleosome modulating activity of shelterin.
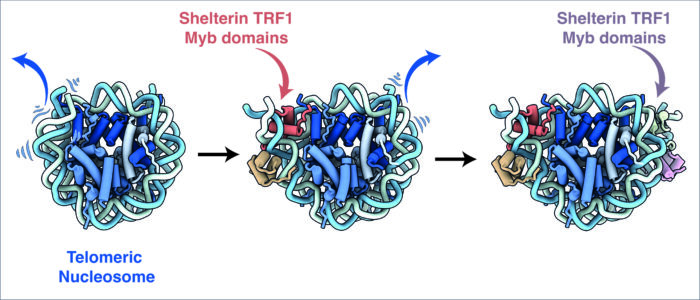
This research also dispels the previous assumption that shelterin associates with telomeres by binding to telomeric DNA. Instead, the new structures illustrate for the first time that TRF1 not only interacts with the nucleosomal DNA, but also with the core histone proteins. Moreover, the group discovered specific phosphorylation of TRF1 is critical for its interactions with histones and nucleosomes. This underscores the role for post-translational modification in shelterin binding to telomeres.
Additionally, it has previously been unclear how shelterin and nucleosomes are organised within telomeres. These structures suggest a stable binding mode of shelterin on telomeric chromatin.
These findings provide a foundation for future studies to further investigate how shelterin and nucleosomes cooperatively form telomeric caps at the ends of chromosomes. The identification of shelterin’s ability to modulate nucleosome also holds important implications as to the role of shelterin in other cellular processes beyond end-capping, such as transcription and replication.
Finally, there is clinical significance to better understanding the mechanism behind telomere development as shortening or damage to telomeres contributes to a range of human diseases, including cancers and premature aging diseases. This new insight into how shelterin and nucleosomes interact will be crucial for further investigations into how telomere dysfunction arises in human diseases.
This work was funded by UKRI MRC, EMBO, the Jane Coffin Childs Memorial Fund for Medical Research and the University of Oxford.
Further references
Structural basis of telomeric nucleosome recognition by shelterin factor TRF1. Hu, H., van Roon, AMM., Ghanim, GE., Ahsan, B., Oluwole, AO., Peak-Chew, SY., Robinson, CV., Nguyen, THD. Science Advances
Kelly’s group page
Carol Robinson’s page
Previous Insight on Research articles
Cryo-EM structures reveal molecular basis of human telomerase recruitment
First atomic model of human telomerase constructed by electron cryo-microscopy