Daily transport of ions in and out of heart cells allows heart rate to anticipate daily demand
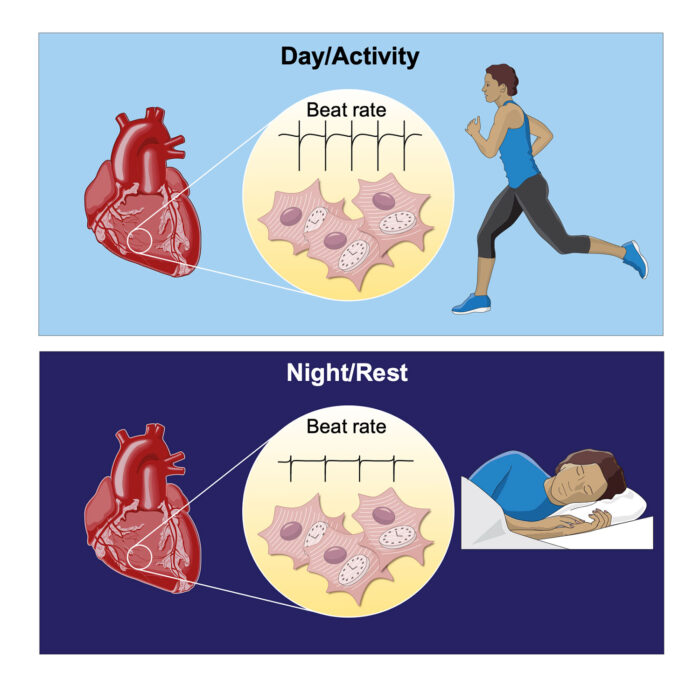
Human physiology is regulated into 24-hour rhythms to anticipate the differing demands of day and night. In the heart, daily variations in the nervous system allow for increased cardiac output (such as elevated heart rate in the morning) – in anticipation of a higher workload as we begin our daily activities. Now, John O’Neill’s group, in the LMB’s Cell Biology Division, working with Peter Newham at AstraZeneca and other collaborators, have shown a mechanism whereby circadian rhythms within heart cells make as great a contribution to daily variation in heart rate as signals from the nervous system.
The study’s lead author, Alessandra Stangherlin, made the initial discovery that cellular ion levels fluctuate to compensate for daily changes in macromolecular crowding of proteins, so that cell volume remains unchanged. During the day, concentrations of cytosolic proteins within cells increase, and ions are exported from the cell to “make room” for them. Across the day, concentrations of potassium, sodium and chloride ions fall by as much as 30% in response to increasing protein levels. To complete the cycle, this process reverses at night when protein levels decrease and ion levels increase. These findings represent a new and important insight into how different cell types maintain homeostasis whilst accommodating changes in cytosolic macromolecular crowding.
To understand the relevance of these findings the team focused on the heart, where the different levels of sodium and potassium inside and outside of cells is what allows the electrical impulse that causes heart cells to contract and so drives the heartbeat. As these sodium and potassium levels change throughout the day to accommodate protein changes, the intrinsic activity of heart cells is forced to vary in a way that allows the heart to better sustain increased beat rate during the daytime in humans, or night-time for nocturnal mice. In both cases the capacity of the heart to sustained increased beat rate is greatest during wakefulness, when fluctuations in demand are typically much greater than during sleep. The study therefore points to how circadian clocks within heart cells help to accommodate daily fluctuations in heart rate.
24 hour rhythms in potassium, sodium and chloride levels osmotically compensate for daily changes in macromolecular crowding to modulate cardiac electrical activity.
This has important clinical value as the disruption of circadian clocks due to lifestyles factors, such as shift work, has been linked to increased incidence of numerous diseases, including arrhythmias and other disorders of the heart. When taken alongside another recent paper that featured findings from John’s group (Hayter et al., Nature Communications, 2021), it now seems very likely that shift workers become more vulnerable to cardiac events due to an uncoupling of clocks in the brain from clocks within heart cells.
It’s also very well known that the elderly are at higher risk of cardiac events in the morning. Findings from this paper suggest that, in the aged, reduced amplitude of daily cardiac sodium and potassium rhythms mean that the heart is less able to accommodate daily changes in demand – i.e. to sustain increased cardiac function in the morning. From a clinical perspective, it is hoped that this new understanding of the mechanisms by which heart cell clocks constrain cardiac capacity may lead to important breakthroughs in cardiovascular medicine, as well as informing preventative measures.
This work is part of the LMB’s and AstraZeneca’s BlueSky collaboration (a research collaboration between AstraZeneca and the Medical Research Council, reference BSF15 and BSF36). It was funded by UKRI MRC, the Human Frontier Science Program, Royal Society of Edinburgh and the Wellcome Trust.
Further References
Compensatory ion transport buffers daily protein rhythms to regulate osmotic balance and cellular physiology. Stangherlin, A., Watson, J.L., Wong, D.C.S., Barbiero, S., Zeng, A., Seinkmane, E., Peak Chew, S., Beale, A.D., Hayter, E.A., Guna, A., Inglis, A.J., Putker, M., Bartolami, E., Matile, S., Lequeux, N., Pons, T., Day, J., van Ooijen, G., Voorhees, R.M., Bechtold, D.A., Derivery, E., Edgar, R.S., Newham, P., O’Neill, J.S., Nature Communications, 12, 6035 (2021). http://doi.org/10.1038/s41467-021-25942-4
John’s group page
Blue Sky Collaboration
UKRI – Scientists uncover circadian rhythm in heart cells
AstraZeneca – How do cellular clocks influence cardiovascular health?
Previous IoRs
How eating feeds into the body clock
Human wound healing is affected by the body clock