Most historical research on immunity has focused on the dedicated cells of our immune system, but, ever since the first single-celled organisms evolved, cells have had to defend themselves against infection. Thus we have a more ancient form of cellular immunity, termed xenophagy, that allows cells throughout our body to capture bacteria that have invaded their cytosol and degrade those invaders inside specialised vesicles termed autophagosomes. Felix Randow’s group, in collaboration with Roger Williams’ group, both in the LMB’s PNAC Division, have now identified how detection of an invading bacterium leads to biogenesis of the autophagosome at the bacterium.
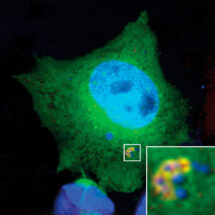
When a Salmonella bacterium initially enters a cell, it is within a membrane bubble called a vacuole. A fraction of the bacteria burst the vacuole membrane and access the cytosol. Should the bacteria go unrecognized they will quickly replicate and take over the cell. The damaged, open membrane has some exposed “eat me” signals that are recognised by the protein galectin 8, which then recruits the autophagy cargo receptor NDP52. Felix’s group had previously identified this pathway and shown that NDP52 delivers the bacterium to an autophagosome. Importantly, this defence mechanism must operate quickly, because if the bacterium is able to divide or move away from the exposed membrane, then the site to which NDP52 recruits the autophagosome won’t be in close enough proximity to stop the infection. However, an outstanding question was whether the autophagosome is initially generated elsewhere in the cell or at the bacterium after detection.
Benjamin Ravenhill and Keith Boyle from Felix’s group used fluorescent microscopy to observe invading Salmonella bacteria in human epithelial cells. To examine this as closely as possible, Benjamin and Keith used a super-resolution microscopy method called Structured Illumination Microscopy (SIM), with the assistance of the LMB’s Light Microscopy facility. This approach allows reconstruction of an image with a resolution greater than is ordinarily possible with light microscopy, permitting a more detailed investigation of the proteins present around an invading Salmonella bacterium. Using these techniques and binding assays, Benjamin and Keith were able to demonstrate that NDP52 binds and recruits one subunit of each of the ULK and TBK1 complexes. These complexes play an important role in biogenesis of the autophagosome, suggesting that initial generation of the autophagosome is localised to the “eat me” signal via NDP52. Indeed, a new study from Richard Youle’s group at the National Institutes of Health in the US, to which Felix’s group also contributed, and featuring in the same issue of Molecular Cell, shows that binding ULK and TBK1 to mitochondria is sufficient to drive their autophagy.
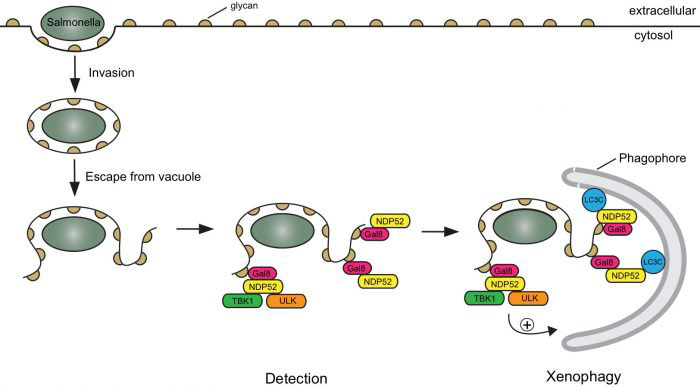
NDP52 has also previously been shown to juxtapose the autophagosome and lysosome in order to drive maturation of the autophagosome and degradation of the ingested bacteria. Felix’s group’s new finding that NDP52 acts at the upstream position of recruiting components required for autophagosome generation, shows that NDP52 is a central regulator of xenophagy of Salmonella bacteria. As xenophagy is a specific form of autophagy, the process by which cells remove damaged internal components, these findings also contribute to increased understanding of autophagy. Defects in autophagy have been associated with Crohn’s disease and osteoarthritis, so improved understanding of the mechanisms involved could lead to development of novel therapies.
The work was funded by the MRC, the Wellcome Trust, and the Jean Shanks Foundation.
Further references:
The cargo receptor NDP52 initiates selective autophagy by recruiting the ULK complex to cytosol-invading bacteria. Ravenhill, BJ., Boyle, KB., von Mühlinen, N., Ellison, CJ., Masson, GR., Otten, EG., Foeglein, A., Williams, R., Randow, F. Molecular Cell, 74: 1-10.
Felix’s group page
Roger’s group page