A delay in the transition from stem cells to neurons allows human brains to grow much larger than brains from other apes
Asking what sets us apart from other animals is a fundamental question with a simple answer: our brains, and, most strikingly, their size. The advent of brain organoid technology has enabled scientists to begin to address this question in more detail due to the capacity of cerebral organoids to model brain development using cells from virtually any species. Madeline Lancaster’s group, in the LMB’s Cell Biology Division, has now identified species-specific differences in early brain development that can help to explain the increased number of neurons in human brains over other apes.
The project started with a simple observation that, mirroring actual brains, human brain organoids were larger than those from other apes. By comparing organoids throughout their development, Silvia Benito Kwiecinski, a PhD student in Madeline’s group, was able to see differences in tissue size and architecture at early stages, before the production of neurons.
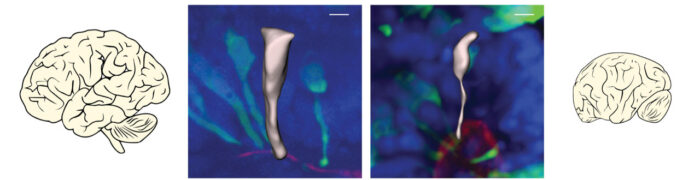
During brain development, neurons are made by cells called neural progenitors. These cells initially have a wide, cylindrical shape that makes it easy for them to split into identical daughter cells with the same shape. This allows expansion of the number of neural progenitors so that more neurons can also be made. Studies in mice have shown that when neural progenitors switch to making neurons, they change not only their identity, but also their shape. In mice, these changes happen simultaneously over a matter of hours, but it’s not known how the transition occurs in the human brain.
By fluorescently labelling individual neural progenitor cells in brain organoids, Silvia could track this transition process and measure differences in timing between human and gorilla brain development. She found that, in apes, this transition takes a long time, occurring over several days, rather than the hours previously seen in mice, and that cell shape is what changes first. In human, neural progenitors were even more delayed in their shape change and, during this time, the human neural progenitors were also dividing more rapidly, allowing production of more daughter neural progenitors and therefore more neurons. This difference in the timing of transition from neural progenitors to neurons could account for a large proportion of the approximate three-fold greater number of neurons seen in human brains than in gorilla or chimpanzee brains.
To look further into what controls this difference in timing, the team compared gene expression over the course of this transition and identified a gene called ZEB2 as a regulator of neural progenitor cell transition. In investigating the role of ZEB2, Stefano Giandomenico, another PhD student in Madeline’s group, with help from Magdalena Sutcliffe, a research support officer, was able to direct early expression of ZEB2 to promote premature transition in human organoids, so that they were more similar to ape organoids. Conversely, pharmacological manipulation allowed the team to delay transition in gorilla organoids, so that they were more similar to human organoids.
The brain is the feature that most clearly sets humans apart from other animals and its increased size is the most obvious difference. This study provides the first insight into the origins of this difference and shows how the specific timing of a simple change in cellular shape early in brain development can have major consequences for diversity between species, even when the basic mechanisms of brain development and the genes involved are so highly conserved.
The work was funded by UKRI MRC, ERC, and CRUK.
Further references
An early cell shape transition drives evolutionary expansion of the human forebrain. Benito Kwiecinski, S., Giandomenico, S., Sutcliffe, M., McDole, K., Freire-Pritchett, P., Lancaster, M. Cell 184, 1-19
Madeline’s group page