Rapid buffering of available water in cells is mediated by assembly and disassembly of intrinsically disordered proteins
Water is the major constituent of cells and critical for life because it provides the driving force for protein folding and many biochemical reactions. The cell interior contains many different biological molecules that directly affect water availability and dynamics within this highly complex, concentrated solution. In addition, cells are continuously bombarded by fluctuations in their external environment that impact water: to survive and thrive, intracellular water balance must be rapidly restored.
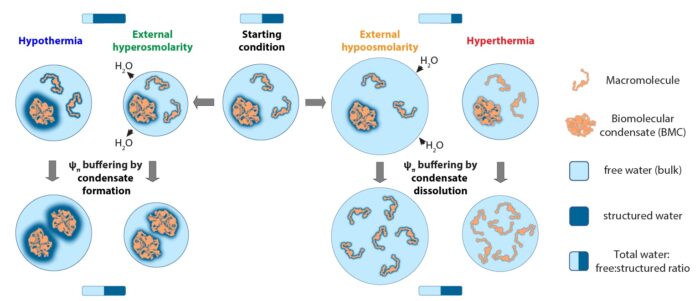
Now, Emmanuel Derivery’s and John O’Neill’s groups in the LMB’s Cell Biology Division, in collaboration with Rachel Edgar’s group at the Imperial College London, have discovered a new mechanism that maintains optimal water availability from second to second. Previously described mechanisms that counteract changes in external salt concentrations, for example, involve bulk movements of water and ions into or out of the cell over much slower timescales (minutes to hours). The team found that water balance inside the cell is rapidly preserved by redistributing biological molecules within fluid assemblies called biomolecular condensates, minimising disruptive changes in volume.
Rapid buffering of water availability is mediated by such macromolecular assembly because cohesive networks of water molecules are disrupted by the addition of biological molecules like proteins, forming ‘hydration layers’ that are crucial for protein structure and function. However, the water constrained within hydration layers cannot move freely and has less potential energy to perform work in the cell. When proteins assemble into higher order structures like biomolecular condensates their surface area exposed to water is reduced, liberating water molecules from hydration layers. Conversely, when proteins dissemble from higher order structures water molecules are sequestered within hydration layers and this reduces overall water availability and energy.
In addition, the team found that water becomes very sensitive to small changes in protein concentration and temperature. The rapid biophysical buffering mechanism they discovered ensures optimum water availability upon natural fluctuations in temperature, salt concentration and pressure that occur countless times in our bodies every day. In crowded solutions like the cytoplasm where there is limited ‘free’ water, even small changes in the distribution or amount of biological molecules has a big impact on water.
Critically, the researchers also found that more and more water molecules are held within hydration layers as temperature falls across the physiological range. The proportion of ‘free’ water in cells is therefore lower at colder temperatures, affecting intracellular water balance in an equivalent way to increasing external salt concentration or pressure, where ‘free’ water leaves the cell. So much so, simply reducing the salt concentration in the culture medium can rescue cells from dying during prolonged exposure to cold (0oC for 24h). In dilute medium, water balance is partially restored as ‘free’ water entering the cell by osmosis offsets the increased amount of water within hydration layers at low temperature. Combining two different stressful conditions, the team could prevent cell death due to their opposing effects on water balance, highlighting its importance for biology.
These concepts were tested using a broad range of experimental systems, including single proteins in a test tube, complex mixtures of biological molecules that exist within the cytoplasm, as well as different physiological models from yeast to human cells. Joseph Watson led the imaging studies and yeast experiments, Estere Seinkmane led proteomic and phosphoproteomic analyses and Christine Styles led the cell viability and cell adaptation experiments.
The study reframes the way we think about phase-separated biomolecular condensates, which are altered in many chronic diseases including neurodegenerative conditions and cancer. In addition, formation of biomolecular condensates is a key feature of many virus infections and our immune responses. By working out what drives biomolecular condensation and its function in cells, the study provides the basis for future investigations into these diseases and development of therapeutics.
Finally, the implications of discovering that human cells can survive prolonged low temperature when cultured in growth medium with lower salt concentration are wide-ranging, from potential treatments for hypothermia to developing better cryopreservation protocols that are critical for preserving fertility during IVF, for example.
This work was funded by UKRI MRC, the Human Frontier Science Program, a Versus Arthritis, Grifols, the Alpha-1-Foundation, the Wellcome Trust, EMBO, Volkswagen ‘Life’, The Royal Society, and the DFG Excellence Cluster Physics of Life.
Further references
Watson, JL., Seinkmane, E., Styles, CT., Mihut, A., Krüger, LK., McNally, KE., Planelles-Herrero, VJ., Dudek, M., McCall, PM., Barbiero, S., Vanden M., Yeu, S., Porebski, BT., Zeng, A., Rzechorzek, NM., Wong, DCS., Beale, AD., Stangherlin, A., Riggi, M., Iwasa, J., Morf, J., Miliotis, C., Guna, A., Inglis, AJ., Brugués, J., Voorhees, RM., Chambers, JE., Meng, Q., O’Neill, JS., Edgar, RS., Derivery, E. Macromolecular condensation buffers intracellular water potential. Nature
Emmanuel’s group page
John’s group page
Rachel’s group page
Previous Insight on Research articles
New method to untangle the contribution of signalling pathways involved in asymmetric cell division