New therapies harness cellular machinery to target tau protein ‘tangles’, which characterise neurodegenerative conditions including Alzheimer’s disease, and significantly improve symptoms of neurodegeneration in mice
Dementia, including Alzheimer’s disease, is predicted to affect one in three people in the UK during their lifetime. Current treatments have a limited effect on memory loss and come with significant side effects. Alzheimer’s disease is pathologically characterised by the accumulation of large filamentous aggregates (‘tangles’) caused by misassembled tau proteins. Leo James’ group, in the LMB’s PNAC Division, have collaborated with Will McEwan’s group at the UK Dementia Research Institute at the University of Cambridge to develop two new therapeutic interventions, which prevent these aggregates from forming, and can even remove pre-existing aggregates. Mice treated with the new therapeutics demonstrated improved motor function..
The new treatments build upon 15-year’s work from Leo’s group on the antiviral protein TRIM21. TRIM21 contains a ‘RING’ domain which clusters around the repetitive structures of capsids found in most viruses. The RING then labels these structures for degradation by the cell’s recycling machinery. The team embarked on this project theorising that if they could redirect the TRIM21 RING to target tau aggregates, then cells would degrade these aggregates as if they were viral capsids. Though other research from the LMB has revealed that tau aggregates can hugely vary in structure, the group reasoned that as they all are made from the same tau protein, they could use tau itself as a targeting sequence.
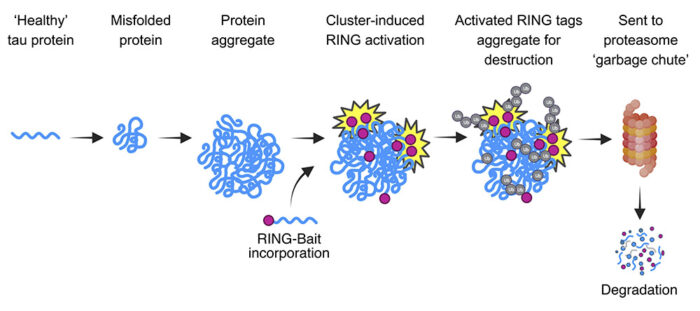
The first therapy, ‘RING-nanobody’, combines a tau-binding nanobody (a small antibody-derived protein roughly one-tenth the size of human antibodies) with TRIM21. The second, ‘RING-Bait’, fuses the RING domain from TRIM21 with tau itself. This means that when tau proteins are incorporated into aggregates, the RING-Bait gets incorporated as well, no matter the aggregate structure. Once incorporated, RING-Bait is activated and causes the entire aggregate to be degraded as it if were a viral capsid.
Postdocs Lauren Miller and Guido Papa initially tested the RING-Bait degrader in cell lines and neurons. Not only did it stop new aggregates from forming, but it also removed pre-existing aggregates. This is crucial in tackling the disease as aggregates are continuously resupplied with new tau proteins. The RING-nanobody treatment showed similarly promising results. Importantly, these tests also demonstrated that the RING-Bait therapy was effective against aggregates induced by Alzheimer’s Disease and progressive supranuclear palsy (PSP) brain extracts. This shows that it is not necessary to know the structure of a tau aggregate or fibril in order to treat the associated disease.
The therapies were next tested in a mouse model of tauopathy. To solve the question of how to deliver treatment into the brain and inside neurons, the group added the DNA sequence containing the information to make the custom proteins into a gene therapy vector, analogous to that used in the Oxford/AstraZeneca COVID-19 vaccine. After delivery, the mice developed significantly fewer aggregates in their brains.
To confirm that fewer aggregates decreased disease development, the group worked with the LMB Workshops to construct a bespoke MouseWalker platform. This platform allowed video tracking of the depressions from the animals paws as they ran across the platform, allowing staff in the LMB Biological Services Group to record motor function with no stress to the mice. Crucially, this showed that mice treated with RING-Bait had significantly improved motor behaviour.

The concept behind RING-nanobody and RING-Bait could be applied beyond tau to widen its application to neurodegenerative diseases caused by other aggregates formed by different proteins. This includes diseases where the aggregate structures are unknown, as these results show it is possible to target the disease-causing aggregates armed only with the knowledge of the protein’s sequence, rather than the structure of the disease-specific aggregate. Additionally, using the ‘bait’ approach means there is no requirement to make antibodies or nanobodies specific to the different fibril structures in each disease.
While this work highlights the removal of aggregates as a viable therapeutic approach to treat neurodegenerative disorders, it is important to stress that the therapies have only shown efficacy in a mouse model of neurodegeneration, and there is a considerable amount of work needed to produce a therapeutic that could treat humans. Aside from verifying its safety and efficacy, researchers must also address the challenge of delivering RING-nanobody and RING-Bait into the human brain. This could utilise cutting-edge gene delivery technology which still requires further development.
This work was funded by UKRI MRC, the Welcome Trust, the Royal Society, the Lister Institute for Preventative Medicine, the UK Dementia Research Institute, Horizon 2020, the European Federation of Pharmaceutical Industries and Associations (EFPIA), Cancer Research UK, the Swiss State Secretariat for Education, Research and Innovation (SERI), Development of Innovative Strategies for a Transdisciplinary approach to Alzheimer’s disease (DISTALZ), and the Agence Natinoale De Lar Recherche (ANR).
Further references
Co-opting templated aggregation to degrade pathogenic tau assemblies and improve motor function. Miller, L.V.C., Papa, G., Vaysburd, M., Cheng, S., Sweeney, P.W. Smith, A., Franco, C., Katsinelos, T., Huang, M., Sanford, S.A.I., Benn, J., Farnsworth, J., Higginson, K., Joyner, H., McEwan, W.A., and James, L.C. Cell
Aggregate-selective removal of pathological tau by clustering-activated degraders. Benn, J., Cheng, S., Keeling, S., Smith, A.E., Vaysburd, M.J., Böken, D., Miller, L.V.C., Katsinelos, T., Franco, C., Dupré., Danis, C., Landrieu, I., Buée, L., Klenerman, D., James, L.C., and McEwan, W.A. Science
Leo’s group page
Will McEwan
UKRI press release
Tau and Alzheimer’s disease
Previous Insight on Research articles
Understanding the protein modifications behind Trim-Away protein degradation technology
HIV-1 needs the cell metabolite IP6 to build its capsid and infect cells
Testing the capacity for intracellular antibodies to neutralise SARS-CoV-2
Animal Research Statement
As a publicly funded research institute, the LMB is committed to engagement and transparency in all aspects of its research. This research used mice, in accordance with the UK Animals (Scientific Procedures) Act 1986. This work was conducted under a Project Licence, reviewed and approved by the MRC Laboratory of Molecular Biology (LMB) Animal Welfare and Ethical Review Body (AWERB) committee and the UK Home Office.
The LMB uses the minimum number of rodents necessary to achieve results and only uses animals in research where there are no suitable alternatives, in line with the 3R’s (replace, reduce, refine). We currently work with fruit flies, nematode worms, mice, rats and zebrafish. More on how the LMB uses animals in research.