 G protein coupled receptor (GPCR) mediated signalling is one of the largest and the most diverse signalling pathways in cellular systems. Human GPCRs sense various signals and activate different Gα proteins to trigger distinct cellular responses. This signalling pathway is important for a broad range of processes such as regulation of the immune system, neurotransmission, vision, taste, and smell. Misregulation of GPCR signalling causes a number of diseases, ranging from cancer and infertility, to various inherited endocrine disorders. Currently, 30% of all prescribed drugs on the market target GPCR signalling. Despite its significance, how GPCRs activate G proteins have remained elusive.
G protein coupled receptor (GPCR) mediated signalling is one of the largest and the most diverse signalling pathways in cellular systems. Human GPCRs sense various signals and activate different Gα proteins to trigger distinct cellular responses. This signalling pathway is important for a broad range of processes such as regulation of the immune system, neurotransmission, vision, taste, and smell. Misregulation of GPCR signalling causes a number of diseases, ranging from cancer and infertility, to various inherited endocrine disorders. Currently, 30% of all prescribed drugs on the market target GPCR signalling. Despite its significance, how GPCRs activate G proteins have remained elusive.
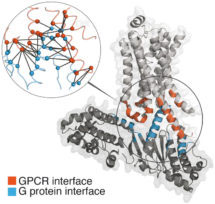
Research led by Madan Babu’s group, in the LMB’s Structural Studies Division, has now revealed the molecular details of this activation mechanism. The group undertook a systematic analysis of over 80 structures and 1000 sequences. They show that despite the enormous diversity of the GPCR signalling system, different GPCRs interact with and activate the different Ga proteins through a highly conserved mechanism. The details of this activation mechanism were verified through mutational studies in collaboration with Dmitry Veprintsev’s group at the Paul-Scherrer Institute (PSI) in Villigen, Switzerland.
Lead researcher Tilman Flock says, “Our work shows how short segments, which undergo disorder-to-order transition, can separate regions important for allosteric activation from the regions required for receptor binding specificity. This might explain how the GPCR-Ga system has diversified rapidly through evolution, while conserving the allosteric activation mechanism.”
The discovery of this universal activation mechanism might enable us to understand why certain mutations that affect GPCR signalling can cause disease and could allow targeted drug design. This work is an important step towards understanding the most medically relevant signalling system in humans.
This research project was funded by the MRC, Boehringer Ingelheim Fonds, Fonds National de la Recherche Luxembourg and the Swiss National Science Foundation.