Identification of new proteostatic mechanism reveals how cells respond to surface perturbations
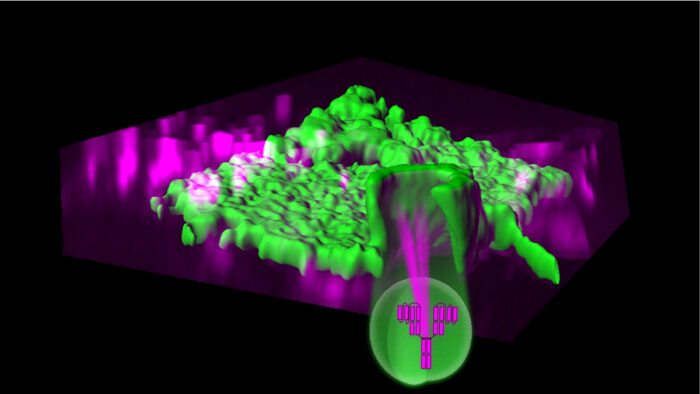
Proteostasis – the process whereby cells maintain protein quality via regulation of protein synthesis, folding, trafficking and degradation – is vital for cellular health. Whilst intracellular protein homeostasis has been well-characterised by past research, understanding of the mechanisms involved in the response to damage of extracellular proteins has thus far proved harder to study owing to a lack of molecular handles. Now, Harvey McMahon’s group, in the LMB’s Neurobiology Division, has collaborated with Andrew Buchanan at AstraZeneca as part of the LMB’s and AstraZeneca’s Blue Sky Collaboration to reveal new insights into plasma membrane proteostasis.
Specifically, the group identified that aggregated cell surface material is internalized by a pathway they named aggregation-dependent endocytosis (ADE). Aggregates can form at cell surfaces in response to physical and chemical stresses which can denature extracellular protein domains. They can also form by crosslinking agents. David Paul, a member of Harvey’s group, used a combination of light microscopy and biochemical assays to study ADE of the biparatopic antibody BS4, targeting the HER2 cell surface receptor in a breast cancer cell line. This antibody was known to be efficiently taken up by cells, but its endocytic route (i.e., the mechanism of uptake) was unknown.
The group found aggregation of extracellular protein domains caused by this antibody as the trigger for internalisation. The endocytosis was found to be mediated by membrane ‘ex’vaginations – as opposed to clathrin-dependent endocytosis which typically starts with ’in’vaginations of the cell membrane. It was also noted that ADE heavily depends on actin polymerization and can result in the formation of larger vesicles, so may be a form of macro-endocytosis.
Crosslinking antibodies selectively ‘activate’ the ADE pathway, as do environmental stresses and other molecules which facilitate aggregation of cell surface proteins. After internalisation of the aggregated cell surface proteins by the ADE pathway, the material is then degraded by lysosomes, the primary digestive unit within cells, thereby helping to maintain plasma membrane homeostasis.
This sheds greater understanding on the mechanisms behind cellular health generally. Moreover, the ADE pathway described here could well be exploited as the entry route used by some viruses or protein aggregates. The group is keen to further explore if ADE is implicated in the cell-to-cell spread of neurotoxic amyloid-like protein aggregates, such as tau or alpha-synuclein, which characterise many neurodegenerative diseases. Additionally, the identification of the molecular determinants of ADE may help influence the design of antibody-based therapies and how to manipulate efficacy.
This work was funded by UKRI MRC, EMBO, and supported through the LMB’s and AstraZeneca’s Blue Sky Collaboration (a research collaboration between AstraZeneca UK Limited and the Medical Research Council, reference BSF10).
Further references
Cell surface protein aggregation triggers endocytosis to maintain plasma membrane proteostasis. Paul, D., Stern, O., Vallis, Y., Dhillon, J., Buchannan, A., McMahon, H. Nature Communications
Harvey’s group page
Andrew Buchanan
Previous Insight on Research articles
Controlling actin polymerisation in clathrin mediated endocytosis