Cryo-EM analysis exposes the structure of aggregated TDP-43, the characterising feature of amyotrophic lateral sclerosis and many types of frontotemporal dementia
Amyotrophic lateral sclerosis (ALS, also known as Lou Gehrig’s disease) is the most common adult-onset motor neuron disease (MND), and frontotemporal dementia (FTD) is the second most common form of presenile dementia following Alzheimer’s disease. ALS and many types of FTD are characterised by the abnormal aggregation of transactive response DNA-binding protein of 43kDa (TDP-43) in the central nervous system. Currently early diagnosis for these diseases is not possible, and there are no disease-modifying therapies available. In part, this is because the structures of pathological TDP-43 aggregates have remained unknown. Now however, Benjamin Ryskeldi-Falcon (formerly Benjamin Falcon) and his group in the LMB’s Neurobiology Division, have worked with collaborators in Japan to determine the structures of aggregated TDP-43 in different brain regions of individuals with ALS and FTD.
Masato Hasegawa and Mari Yoshida from the Department of Brain and Neurosciences, Tokyo Metropolitan Institute of Medical Science and the Institute for Medical Science of Aging, Aichi Medical University respectively, provided Benjamin’s group with brain samples containing aggregated TDP-43 taken from the frontal and motor cortices of individuals afflicted with ALS and FTD. Diana Arseni, a postdoc in Benjamin’s group, performed electron cryo-microscopy (cryo-EM) on the samples and, using helical reconstruction, exposed their structures up to resolutions of 2.6 angstrom (Å).
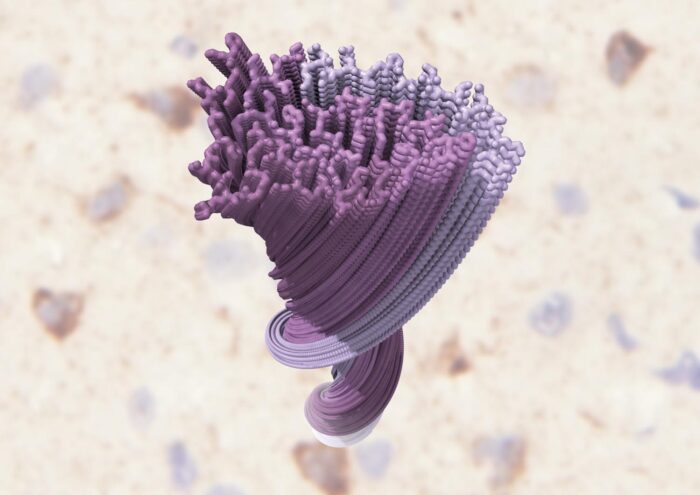
High-resolution electron cryo-microscopy structure of pathological TDP-43 filaments from amyotrophic lateral sclerosis with frontotemporal lobar degeneration showing the double-spiral-shaped filament fold formed by the low-complexity domain of TDP-43.
Analysis of these structures by Diana, Alexey Murzin and Benjamin revealed an amyloid-like helical filament structure comprised of stacked TDP-43 molecules. Interestingly, the structured filament core adopts a previously unseen double-spiral-shaped fold perpendicular to the helical axis that was conserved across all examined brain regions and individuals, but which shows no similarity with TDP-43 filaments previously formed in experimental model systems.
Additionally, the team discovered that the filament core is formed by a region of TDP-43 with a low-complexity amino acid composition. This results in filament surfaces that are structurally and chemically unique in comparison to those of other neurodegenerative disease-associated filaments formed by proteins such as tau and alpha-synuclein (α-synuclein). This finding led the group to consequently observe distinct molecules of presently unknown chemical identity interacting with the surfaces of the filaments in their structure in comparison to other neurodegenerative disease-associated filament structures. Overall, these results suggest that the molecular disease-mechanisms of ALS and FTD with TDP-43 aggregates may differ from those of other neurodegenerative diseases.
The determination of the structure of aggregated TDP-43 and how it differs from amyloid filaments in other neurodegenerative diseases provides a possible explanation for the poor efficacy of current diagnostic approaches, which rely on tracer compounds developed to bind to amyloid filaments. Importantly, in showing that a single TDP-43 filament structure characterises both ALS and FTD, the study posits that this could be a viable therapeutic target and also highlights possible alternative binding sites in the filament which could be targeted for more effective diagnostic agents.
This research relied on individuals (and their families) donating their brains to research.
This work was funded by UKRI MRC, Alzheimer’s Research UK, the Japan Agency for Medical Research and Development (AMED) and the Japan Science and Technology Agency (JST).
Further references
Structure of pathological TDP-43 filaments from ALS with FTLD. Arseni, D., Hasegawa, M., Murzin, AG., Kametani, F., Arai, M., Yoshida, M., Ryskeldi-Falcon, B. Nature
Benjamin’s group page
Masato Hasegawa’s Dementia Research Project at the Tokyo Metropolitan Institute of Medical Science
Nature News and Views