Analysis of GABAA receptors reveals potential for tens of thousands of subunit arrangements with significant therapeutic implications
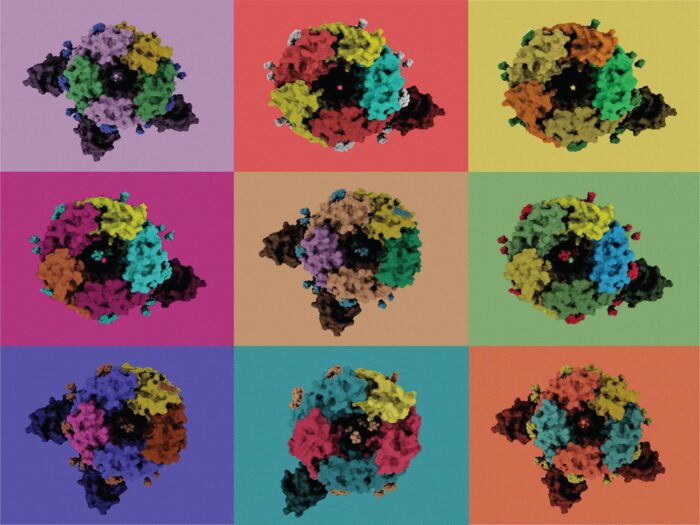
Type A gamma-aminobutyric acid receptors (GABAARs) are the principal mediators of inhibitory neurotransmission in vertebrates, which is crucial for the normal function of the central nervous system. GABAARs also operate in many other tissues, such as to assist hormonal release in the pancreas, and dampen the inflammatory response of the immune system. GABAAR dysfunction is implicated in numerous neurological and neuropsychiatric disorders and, as such, these receptors have proved successful drug targets. Despite the fundamental importance of GABAARs, molecular understanding of these receptors is surprisingly limited. GABAARs are pentameric ligand-gated chloride channels. Their subunits can be encoded by 19 paralogous genes however only a small number of pentameric arrangements have been observed experimentally. Now, Radu Aricescu’s group, in the LMB’s Neurobiology Division, who investigate the structure, physiology and pharmacology of GABAARs, have found that they are far more diverse than previously assumed.
Using cryogenic electron microscopy (cryo-EM), Andrija Sente, a PhD student in Radu’s group, aimed to solve the structure of a representative extrasynaptic GABAAR. During data processing, he discovered that that the arrangement of its subunits differed from the expected “canonical” GABAAR stoichiometry. Further analysis found that cells expressing α4, β3 and δ or α4, β3 and γ2 subunits assemble multiple receptor populations in a differential, context-dependent, but non-random manner.
The discovery of receptors with β3/β3 subunit interfaces was especially interesting as the group has previous determined that histamine can occupy the agonist site here. Histamine is a neurotransmitter which is co-released with GABA in the brain by a subset of neurons involved in regulating the sleep/wake cycle. Using electrophysiology, Rooma Desai in Keith Miller’s group at the Massachusetts General Hospital, Boston confirmed that GABAARs with one α4 and three (or four) β3 subunits can function as ‘coincidence detectors’, modulated by both GABA and histamine.
Andrija, Jonas Miehling, another PhD student in Radu’s group, and Tomas Malinauskas from the Wellcome Centre for Human Genetics, University of Oxford, have also solved structures of receptors bound to two drug-like molecules: gaboxadol, a candidate for treatment of insomnia, and Ro15-4513, an intriguing antidote of alcohol inebriation. The team discovered that gaboxadol – a synthetic agonist of α4β3δ receptors – binds at multiple subunit interfaces types, whereas Ro15-4513, contrary to previous hypotheses, cannot bind the extrasynaptic α4β3δ or α4β3 receptors. Instead, Ro15-4513 binds α1β3γ2 and α4β3γ2 synaptic types. These findings help to better understand the impact of subunit-dependent drug actions across GABAARs.
A limitation the team faced in their study was that it was only possible to solve the structures of receptors that could be purified and classified. To maximise the applicability of the study, Andrija mined single-cell RNA sequencing results from the human cortex. Surprisingly, he found that most neuron types express sufficiently large subsets of these genes to assemble potentially tens or even hundreds of thousands of receptors with different subunit arrangements.
To overcome the limitations of cryo-EM, Andrija worked with Katerina Naydenova, a PhD student in Chris Russo’s group in the LMB’s Structural Studies Division. They designed a computational strategy which simulated the distribution of receptor subtypes given two sets of parameters; the subunit abundances and the relative interface likelihoods. With conditions mimicking the distribution of receptors as observed in the group’s study, the programme consistently predicted the existence of additional subtypes, with distinct signalling properties.
The findings from this study present a new way of looking at the field of molecular neuroscience in general. Other oligomeric receptors, encoded by multiple genes just like GABAAR, may also be more diverse than currently thought, and consequently lead to more diverse signalling outputs. This diversity needs to be explored and harnessed in order to develop drugs with better specificity and fewer side effects.
GABAA receptors are among the most important human drug targets for a range of neurological and neuropsychiatric disorders, including anxiety, depression, insomnia, epilepsy and schizophrenia. This research highlights the potential to adapt clinical treatments to target ligand-binding sites of a specific receptor arrangement, so that drugs could have increased specificity and fewer side effects. These receptors are also the major targets of volatile and intravenous general anaesthetics, which are essential to modern medicine and surgery. Moreover, as GABAARs are also involved in signalling outside the central nervous system, the impact of this research holds therapeutic potential for other conditions such as diabetes and cancer immunotherapy.
The work was funded by UKRI MRC, National Institute for General Medical Sciences, Massachusetts General Hospital, Cambridge Trust, University of Cambridge, Boehringer Ingelheim Fonds and the Wellcome Trust.
Further references
Differential assembly diversifies GABAA receptor structures and signalling. Sente, A., Desai, R., Naydenova, K., Malinauskas, T., Jounaidi, Y., Miehling, J., Zhou, X., Masiulis, S., Hardwick, SW., Chirgadze, DY., Miller, KW., Aricescu, AR. Nature
Radu’s group page
Chris’ group page
Keith Miller
Tomas Malinauskas
Previous Insight on Research articles
Atomic advance for cryo-EM
A synthetic molecule that can restore lost connections in the brain
Structures of the human GABAA receptor reveal how it functions and could help improve key drugs