Cryo-EM reveals that TDP-43 forms amyloid filaments with a new fold in the most common type of frontotemporal lobar degeneration
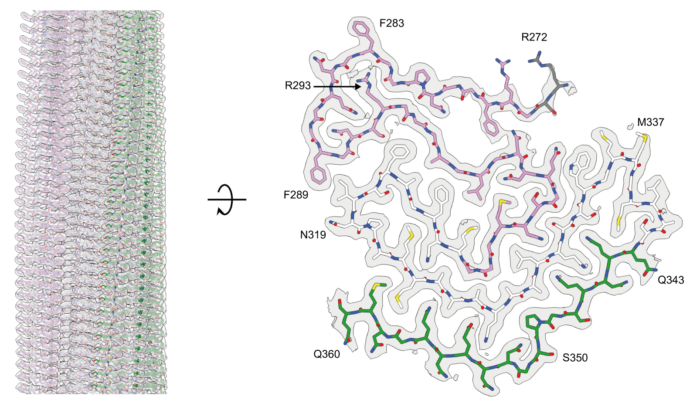
The abnormal assembly of TAR DNA-binding protein 43 (TDP-43) is believed to be responsible for almost all cases of amyotrophic lateral sclerosis (ALS) and around half of cases of frontotemporal dementia (FTD). At least four types of frontotemporal degeneration with TDP-43 pathology (FTLD-TDP) are defined by distinct brain distributions of assembled TDP-43 and lead to differing clinical presentations of FTD. New research from Benjamin Ryskeldi-Falcon’s group in the LMB’s Neurobiology Division has revealed the structures of assembled TDP-43 from the brains of individuals with type A FTLD-TDP, the most common variation.
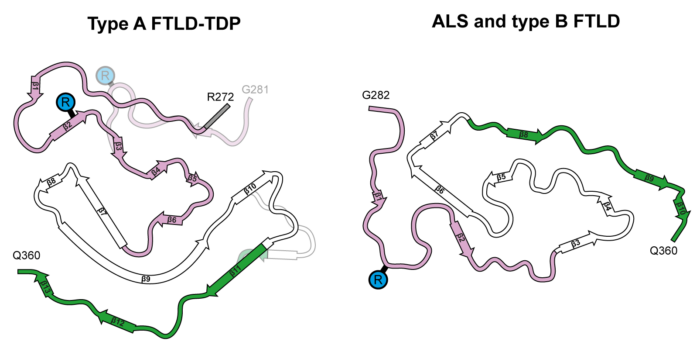
The group’s structures revealed that TDP-43 forms amyloid filaments with a characteristic fold in type A FTLD-TDP. This resolves the longstanding debate of whether TDP-43 forms amyloid filaments in diseases beyond ALS. The filament fold is distinct from the TDP-43 fold in ALS and type B FTLD-TDP, as previously determined by Benjamin’s group, thereby establishing that distinct filament folds of TDP-43 characterise different neurodegenerative conditions.
Using electron cryo-microscopy (cryo-EM), lead author Diana Arseni, a postdoc in Benjamin’s group, was able to determine these structures from brain samples provided by Masato Hasegawa at the Tokyo Metropolitain Institute of Medical Science and Bernardino Ghetti at the Indiana University School of Medicine. The cryo-EM analysis also demonstrated the existence of structural variation within individual TDP-43 filaments. This shows that amyloid filaments do not always adopt uniform repetitive structures, which has implications for the development of diagnostic and therapeutic tools that target these filaments.
The authors observed the unusual burial of arginine residues without charge compensation. Mass spectrometry analysis by Renren (Heidy) Chen, a PhD student in Benjamin’s group, led to the identification of two new post-translational modifications of TDP-43: citrullination and mono-methylation of arginine. Citrullination may facilitate the burial of arginine residues, whereas mono-methylation may mediate structural variation at this site. These results suggest a role for post-translational modifications in the formation and structures of TDP-43 amyloid filaments.
Currently, there are no effective disease-modifying therapies for ALS or FTD, and no way to diagnose these diseases early enough to allow for impactful therapeutic intervention. This research will guide studies into the molecular mechanisms of TDP-43 assembly, as well as the development of tools that target distinct TDP-43 filament folds for early diagnosis and, possibly, treatment of ALS and FTD.
This work was funded by UKRI MRC, Alzheimer’s Research UK, US National Institutes of Health, Japan Agency for Medical Research and Development (AMED), Japan Science and Technology Agency (JST), the Cambridge Commonwealth, European and International Trust, and the Leverhulme Trust.
Further references
TDP-43 forms amyloid filaments with a distinct fold in type A FTLD-TDP. Arseni, D., Chen, R., Murzin, AG., Peak-Chew, SY., Garringer, HJ., Newell, KL., Kametani, F., Robinson, AC., Vidal, R., Ghetti, B., Hasegawa, M., Ryskeldi-Falcon, B. Nature
Benjamin’s group page
Masato Hasegawa, Tokyo Metropolitan Institute of Medical Science, Japan
Bernardino Ghetti, Indiana University School of Medicine, USA
Previous Insight on Research articles
Structural breakthrough in study of aggregated TDP-43 protein in brains of ALS and FTD sufferers