Electron cryomicroscopy reveals the surface layer structure of the extremophilic bacterium, Deinococcus radiodurans
Deinococcus radiodurans is the “toughest bacterium known” due to its remarkable ability to tolerate various environmental stresses, including nuclear radiation, extreme temperatures, vacuum, or oxidation. It can even survive for years in outer space.
Its ability to resist extreme conditions is due in part to its complex cell envelope, which is encapsulated within a hyperstable surface layer (S-layer). However, despite decades of research the structural details of this extremophile’s cell surface have remained obscure. Now a new study by Tanmay Bharat’s group in the LMB’s Structural Studies Division, in collaboration with Vikram Alva from the Max Plank Institute in Tübingen, reports the cryo-EM structure of the S-layer of D. radiodurans and reveals that it is formed by the self-assembly of a protein containing immunoglobulin domains in a highly interconnected arrangement.
Using electron cryotomography (cryo-ET), a technique that combines the power of 3D molecular-level imaging with the best structural preservation that is currently possible to achieve, the group obtained a model of the native arrangement of the S-layer on cells. The study, led by PhD student Andriko von Kügelgen, confirms that the D. radiodurans S-layer is composed exclusively of a protein called Hexagonally Packed Intermediate-layer (HPI), arranged in a planar hexagonal lattice forming an array of immunoglobulin-like folds. The structure of the D. radiodurans S-layer also reveals how this sheet-like assembly displays hyperstability. The structure shows that each monomer extends into the adjacent hexamer, resulting in a highly interconnected and stable, structure that would require multiple biochemical steps for complete disassembly. Additionally, the lattice is stabilized by many salt bridges that provide exceptional stability even in the presence of strong denaturing detergents and high temperatures.
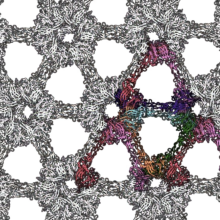
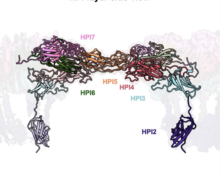
Furthermore, this S-layer shares similarities with immunoglobulin domain-containing S-layers from distantly related organisms, highlighting common features in cell surface organization across different domains of life. Such cell surface molecules may have supported the evolution of eukaryotic recognition systems based on immunoglobulin domains, and the human immune system could have evolved from their prokaryotic counterparts.
This hyperstable S-layer structure of D. radiodurans could be exploited for applications in synthetic biology, such as surface presentation of molecules. Also, this structural knowledge could be used to make large scale protective materials against radiation, heat and other chemicals.
This work was supported by UKRI MRC, the Human Frontier Science Program, the Vallee Research Foundation, EMBO, the Leverhulme Trust, the Lister Institute for Preventative Medicine, a Natural Sciences and Engineering Research Council of Canada Discovery Grant, an NSERC Postdoctoral Fellowship, and the Max Planck Society.
Further references
Interdigitated immunoglobulin arrays form the hyperstable surface layer of the extremophilic bacterium Deinococcus radiodurans. von Kügelgen, A., van Dorst, S., Yamashita, K., Sexton, D.L., Tocheva, E.I., Murshudo, G., Alva, V., and Bharat, T.A.M. PNAS.
Tanmay Bharat group page.