Cryo-EM study reveals structure of new protein responsible for formation of amyloid filaments in human brains
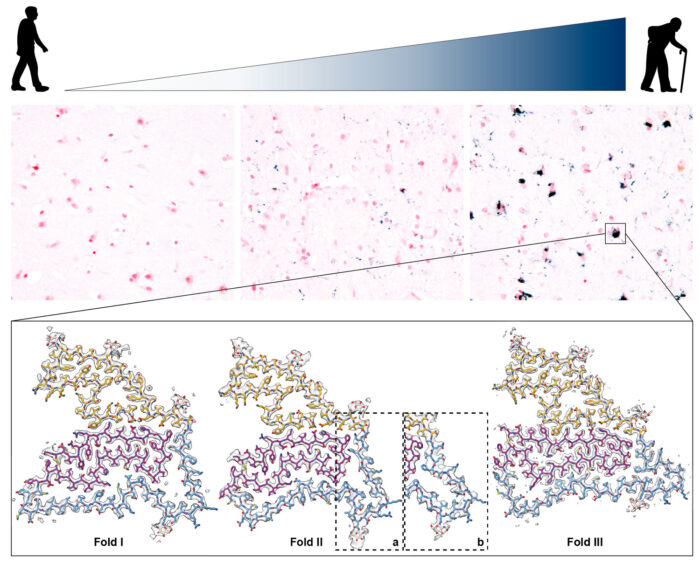
The assembly into filaments of a range of proteins – including tau, amyloid-beta, alpha-synuclein and TDP-43 – is the defining characteristic of most neurodegenerative diseases. Building upon previous collaborative studies of these proteins, Sjors Scheres and Michel Goedert’s groups have now identified TMEM106B as a new protein which forms amyloid filaments in human brains.
In examining the brains of 22 individuals with a wide range of neurodegenerative diseases, the team discovered amyloid filaments made of the intralumenal domain of the lysosomal transmembrane protein TMEM106B. Using cryo-electron microscopy (cryo-EM), lead authors Manuel Schweighauser, Diana Arseni, Melissa Huang, Sofia Lövestam, Yang Shi and Yang Yang determined their atomic structures.
Interestingly, they discovered three distinct folds with no clear relationship to disease. This is a departure from previous studies of tau, amyloid-beta, and alpha-synuclein filaments, which showed specific structures in many neurodegenerative diseases.
Further investigation into brains from neurologically normal controls led to the discovery of TMEM106B filaments in older, but not younger, individuals. This suggests that these filaments form in an age-dependent manner, that they thus may affect the progression of disease, but that they are probably not causative.
This research highlights the ability of cryo-EM to discover previously unknown filaments. The findings point to future research possibilities in investigating the role of TMEM106B filaments to disease development. More broadly, this paper furthers our understanding of neurodegenerative diseases and thus holds clinical implications for diagnostic and treatment approaches.
The study was supported by Benjamin Ryskeldi-Falcon and Alexey Murzin at the LMB, Mehtap Bacioglu and Maria Grazia Spillantini at the Department of Clinical Neurosciences, University of Cambridge who performed immunohistochemistry, Masato Hasegawa from Tokyo Metropolitan Institute of Medical Science and Bernardino Ghetti from the Department of Pathology and Laboratory Medicine, Indiana University School of Medicine, who provided brain samples.
The work was funded by UKRI MRC, the European Federation of Pharmaceutical Industries and Associations’ Innovative Medicines Initiative, the Japan Agency for Science and Technology, the Japan Agency for Medical Research and Development, the Japan Society for the Promotion of Science, the U.S. National Institutes of Health, the Department of Pathology and Laboratory Medicine, Indiana University School of Medicine, the Safra Foundation, the Rossy Foundation, the National Institute for Health Research Queen Square Biomedical Research Unit in Dementia, the National Center of Neurology and Psychiatry, Alzheimer’s Research UK and the Reta Lila Weston Institute for Neurological Studies.
Further references
Age-dependent formation of TMEM106B amyloid filaments in human brains. Schweighauser, M., Arseni, D., Bacioglu, M., Huang, M., Lövestam, S., Shi, Y., Yang, Y., Zhang, W., Kotecha, A., Garringer, HJ., Vidal, R., Hallinan, GI., Newell, KL., Tarutani, A., Murayama, S., Miyazaki, M., Saito, Y., Yoshida, M., Hasegawa, K., Lashley, T., Revesz, T., Kovacs, GG., van Swieten, J., Takao, M., Hasegawa, M., Ghetti, B., Spillantini, MG., Ryskeldi-Falcon, B., Murzin, AG., Goedert, M., Scheres, SHW. Nature
Sjors’ group page
Michel’s group page
Benjamin’s group page
Alexey’s group page
Maria Grazia Spillantini’s page
Bernardino Ghetti’s page
Masata Hasegawa’s page
Indiana University School of Medicine – Researchers discover new information about amyloid filaments in neurodegenerative diseases
Previous Insight on Research articles
Atomic structures of Ab42 filaments from the brains of individuals with Alzheimer’s disease and other neurodegenerative conditions
Structural breakthrough in study of aggregated TDP-43 protein in brains of ALS and FTD sufferers
Classification of human tauopathies based on tau filament folds
First structures of α-synuclein filaments from human brain
A novel tau fold in the neurodegenerative disease corticobasal degeneration